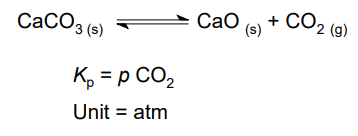Chemical Equilibrium
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
What is Kc and Kp?
Kc = Equilibrium constant in terms of concentration
Kp = Equilibrium constant in terms of partial pressure
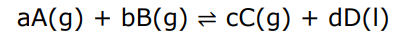
Write the expression of Kc
Liquid D is omitted as its concentration does not change.
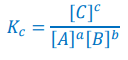
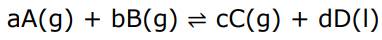
Write the expression for Kp
Liquid D is omitted as it has no partial pressure
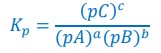
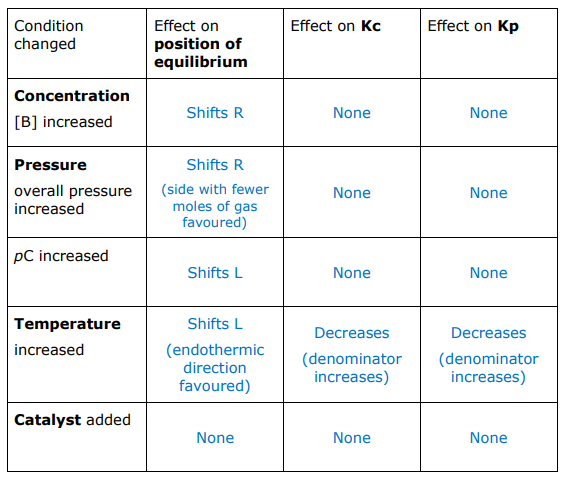
Fill in this table for the equation:
aA(g) + bB(g) ⇌ cC(g) + dD(l)
ΔH –ve
(a + b) > (c + d)
Sulfur dioxide reacts with oxygen to form sulfur trioxide in a reversible reaction.
2SO2(g) + O2(g) ⇌ 2SO3(g)
Write an expression for the equilibrium constant, Kp, of this reaction.
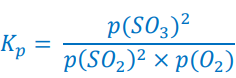
2SO2(g) + O2(g) ⇌ 2SO3(g)
Industrially, the conversion of sulfur dioxide and oxygen into sulfur trioxide is carried out at slightly above atmospheric pressure.
Suggest why this condition is chosen.
An increase in pressure favours the forward reaction (because the RHS has fewer gaseous moles) and speeds up the rate of reaction
A pressure only slightly above atmospheric pressure does not require too much energy to maintain and is not too hazardous.
A chemist investigated methods to improve the synthesis of sulfur trioxide from sulfur dioxide.
2SO2(g) + O2(g) ⇌ 2SO3(g)
The chemist:
mixed together 1.00mol SO2 and 0.500mol O2 with a catalyst at room temperature
compressed the gas mixture to a volume of 250cm3
allowed the mixture to reach equilibrium at constant temperature without changing the total gas volume.
At equilibrium, 82.0% of the SO2 had been converted into SO3
Determine the concentrations of SO2, O2 and SO3 present at equilibrium and calculate Kc for this reaction, including its units
Kc = [SO3]2/[SO2]2[O2]
Kc = [3.28]2/[0.70]2[0.36]
57.6 mol-1dm3
Must have 3sf
![<p>K<sub>c</sub> = [SO<sub>3</sub>]<sup>2</sup>/[SO<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>]</p><p></p><p>K<sub>c</sub> = [3.28]<sup>2</sup>/[0.70]<sup>2</sup>[0.36]</p><p></p><p>57.6 mol<sup>-1</sup>dm<sup>3</sup></p><p>Must have 3sf</p>](https://knowt-user-attachments.s3.amazonaws.com/bd76fbeb-9302-48a0-942e-c11d6746f760.png)
Explain what would happen to the pressure as the system was allowed to reach equilibrium.
2SO2(g) + O2(g) ⇌ 2SO3(g)
Pressure decreases as there are fewer moles of gaseous products
The chemist repeated the experiment at the same temperature with 1.00mol SO2 and an excess of O2.
The gas mixture was still compressed to a volume of 250cm3
State and explain, in terms of Kc, how the equilibrium yield of SO3 would be different from the yield in the first experiment above.
Kc does not change (with pressure / concentration).
If [O2] increases, the denominator of the Kc expression is greater, the yield of SO3 (the numerator) must increase to keep Kc constant.
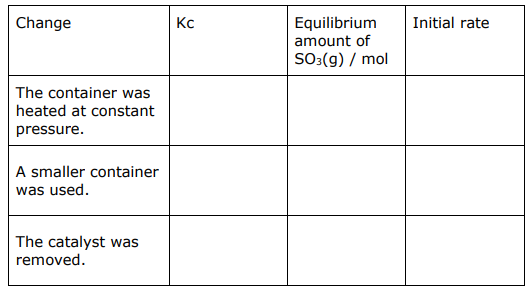
The chemist repeats the experiment three further times. In each experiment, the chemist makes one change, but uses the same initial amounts of SO2 (1.00mol) and O2 (0.500mol).
Complete the table to show the predicted effect of each change compared with the original experiment.
The reaction is exothermic
Increasing concentration effect on equilibrium
If the reactant concentration is increased, the POE will shift to the right (towards the product) to use up more reactant
If the production concentration is increased, the POE will shift to the left (towards the reactant)
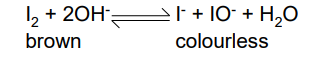
Increasing the concentration of OH- ions
The PO will shift to the right, giving a higher yield of I- and IO-
The colour would change from brown to colourless
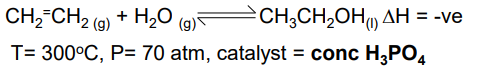
Ethene to Ethanol
Low temp gives good yield but slow rate
High pressure gives good yield and high rate, but too high pressure leads to high energy costs for pumps to produce the pressure
High pressure also leads to unwanted polymerisation of ethene to polyethene
Contact process
Low temp gives good yield but slow rate
High pressure gives slightly better yield and high rate, but too high pressure leads to high energy costs for pumps to produce the pressure
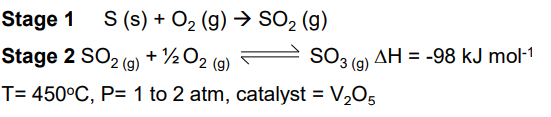
Haber process
Low temp gives good yield but slow rate
High pressure gives slightly better yield and high rate, but too high pressure leads to high energy costs for pumps to produce the pressure
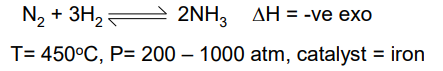
Production of methanol from CO
Low temp gives good yield but slow rate
High pressure gives slightly better yield and high rate, but too high pressure leads to high energy costs for pumps to produce the pressure
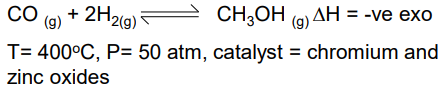
Effects of catalysts on equilibrium
A catalyst has no effect on the position of equilibrium, but it will speed up the rate at which the equilibrium is achieved. It does not effect the position of equilibrium because it speeds up the rates of the forward and backward reactions by the same amount.
What does Kc only change with?
Kc only changes with temperature.
It does not change if pressure or concentration is altered. A catalyst has no effect on Kc.
Value of Kc changing
The larger the Kc, the greater the amount of products.
If Kc is smaller, the equilibrium favours the reactants
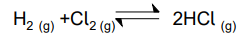
In a container of volume 600cm3 there were initially 0.500mol of H2 and 0.600 mol of Cl2 .
At equilibrium there were 0.200 mol of HCl.
Calculate Kc
ICEE Table (Initial moles, Change in moles, Equilibrium moles, Equilibrium concentration)
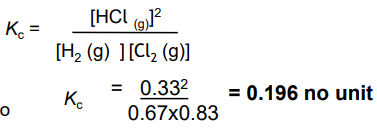
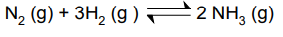
Initially there were 1.5 moles of N2 and 4 mole of H2, in a 1.5 dm3 container.
At equilibrium 30% of the Nitrogen had reacted.
Calculate Kc
30% of the nitrogen had reacted
30% x 1.5 = 0.45 moles reacted.
3 x 0.45 moles of H2 must have reacted and 2 x 0.45 moles of NH3 must have formed
Moles of reactant at equilibrium = Initial moles - moles reacted
Moles of N2 at equilibrium = 1.5 - 0.45 = 1.05
Moles of H2 at equilibrium = 4.0 - (0.45 × 3) = 2.65
Moles of NH3 at equilibrium = 0 + (0.45 × 2) = 0.9
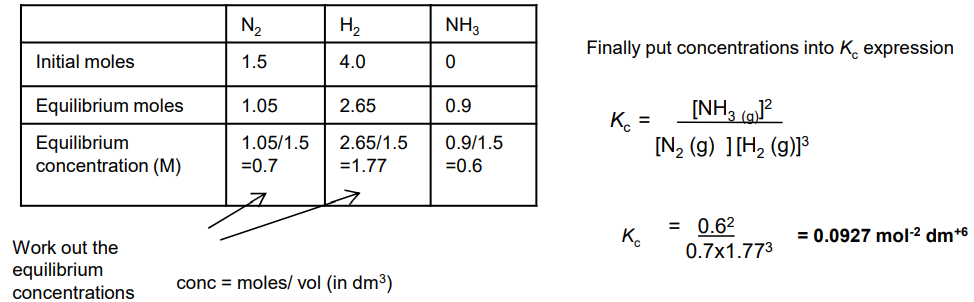
What is the partial pressure of a gas?
The pressure that the gas would have, if it alone occupied the volume occupied by the whole mixture.
If a mixture of gases contains 3 different gases then the total pressure will equal the 3 partial pressure added together
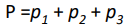
Mole fraction
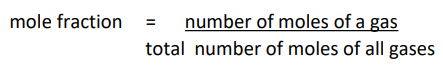
Mole fraction for a 3 part mixture
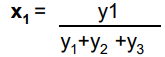
How to calculate the partial pressure of a gas
Calculate mole fraction of the gas (moles/total moles)
Then multiply the mole fraction by the total pressure provided (Mole fraction of gas 1 x total pressure) to calculate the partial pressure of that gas
A mixture contains 0.2 moles N2, 0.5 moles O2 and 1.2 moles of CO2.
If the total pressure is 3atm, calculate the partial pressures of the 3 gases
Calculate molar fraction
Mole fraction x pressure
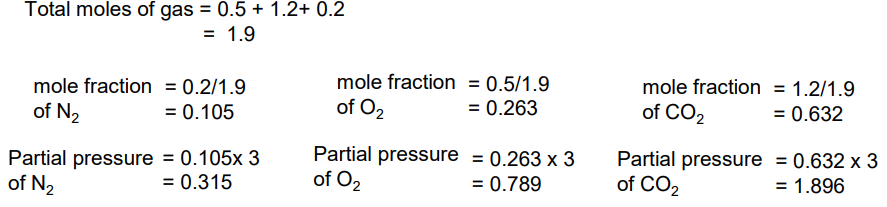
Units of Kp
atm
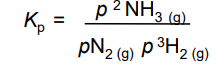
What’s the units of
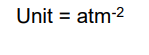
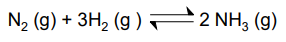
1 mole of N2 and 3 moles of H2 are added together and the mixture is allowed to reach equilibrium.
At equilibrium 20% of the N2 has reacted.
If the total pressure is 2atm what is the value of Kp?
ICEMP Table
Initial moles
Change in moles
Equilibrium moles
Molar fraction
Partial pressure
Answer = 0.0469 atm2
Rules for writing Kp expression
Kp expressions only contain gaseous substances. If the equation has a substance with another state, it is not included in the expression.