Respiratory Principles
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
what are the two basic functions of the respiratory system?
o Maintain O2 supply from atmosphere to tissues
o Eliminate CO2 (and other metabolic products) from tissue to atmosphere
= Respiratory Gas Exchange
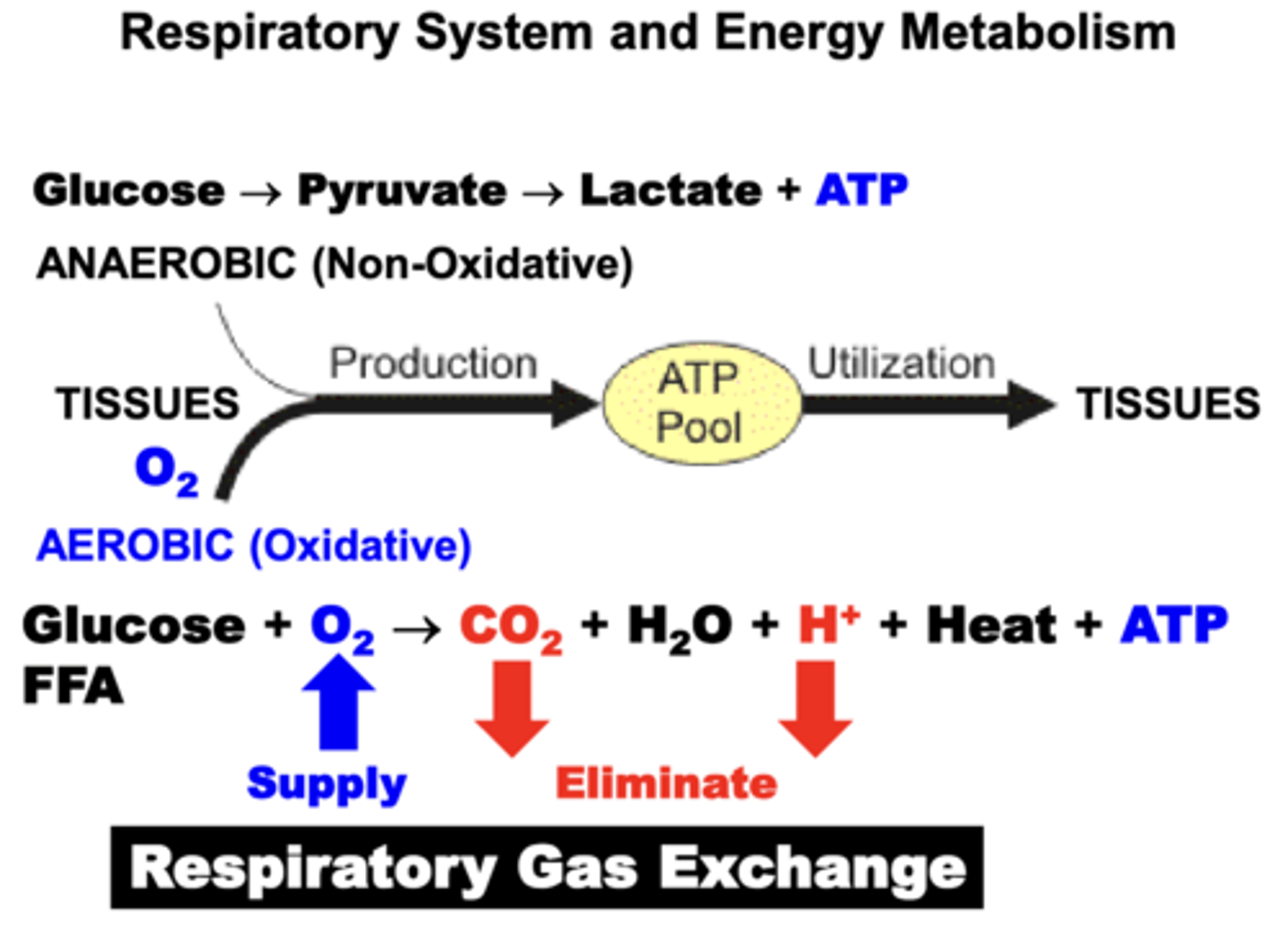
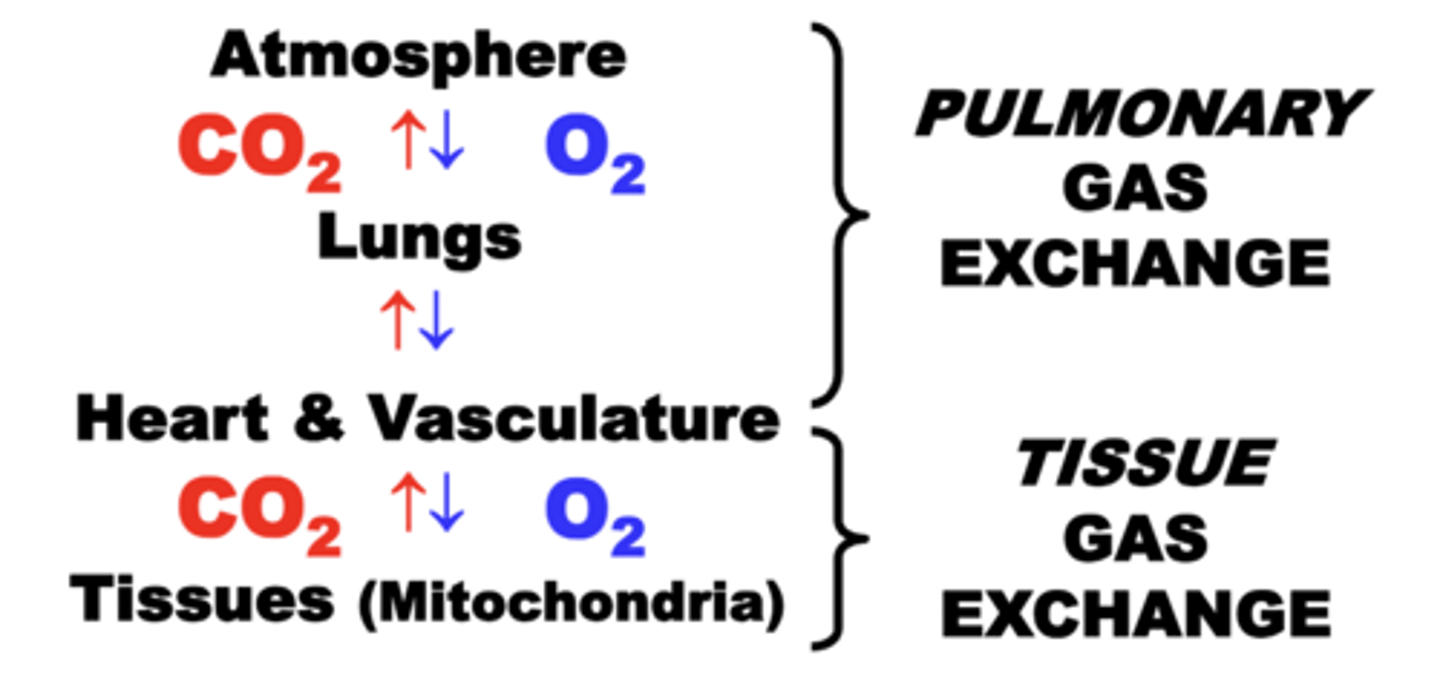
Pulmonary Gas Exchange
Movement of O2 and CO2 between atmosphere, lungs, and heart & vasculature.
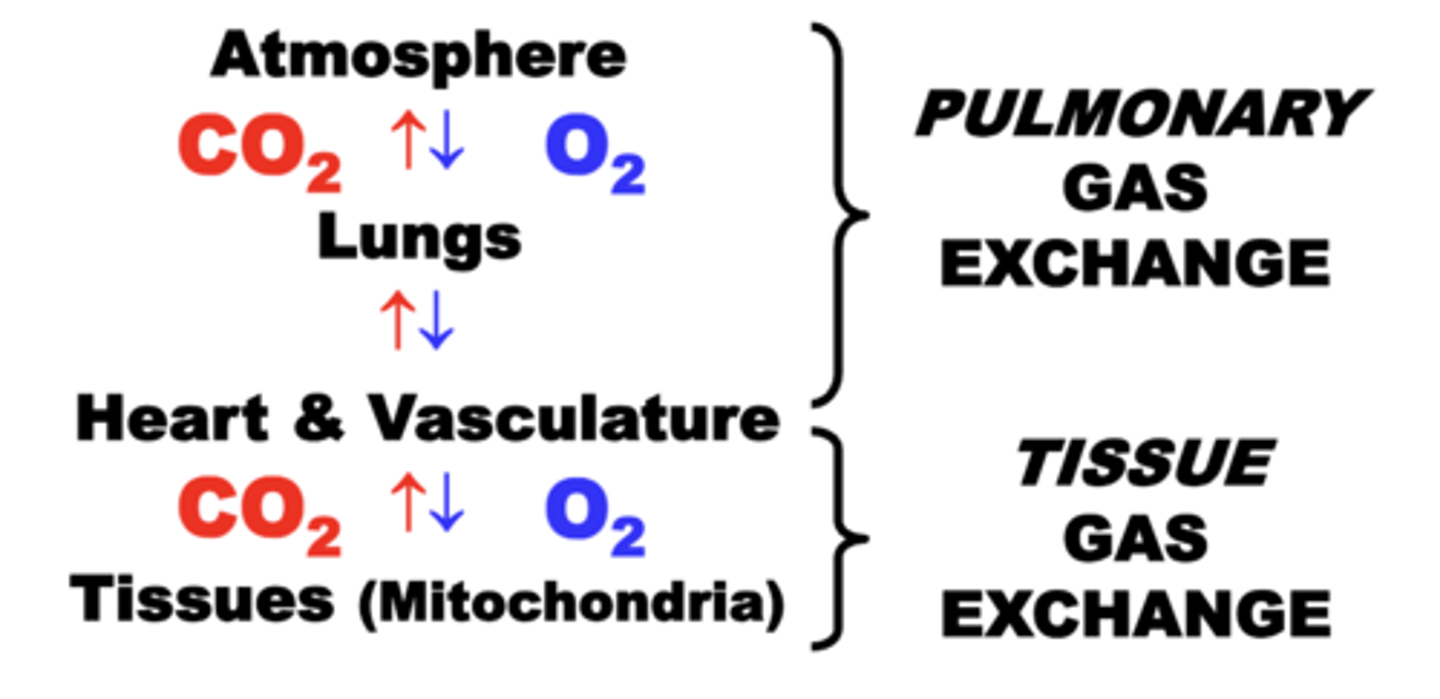
Tissue Gas Exchange
Movement of O2 and CO2 between heart & vasculature, and tissues.
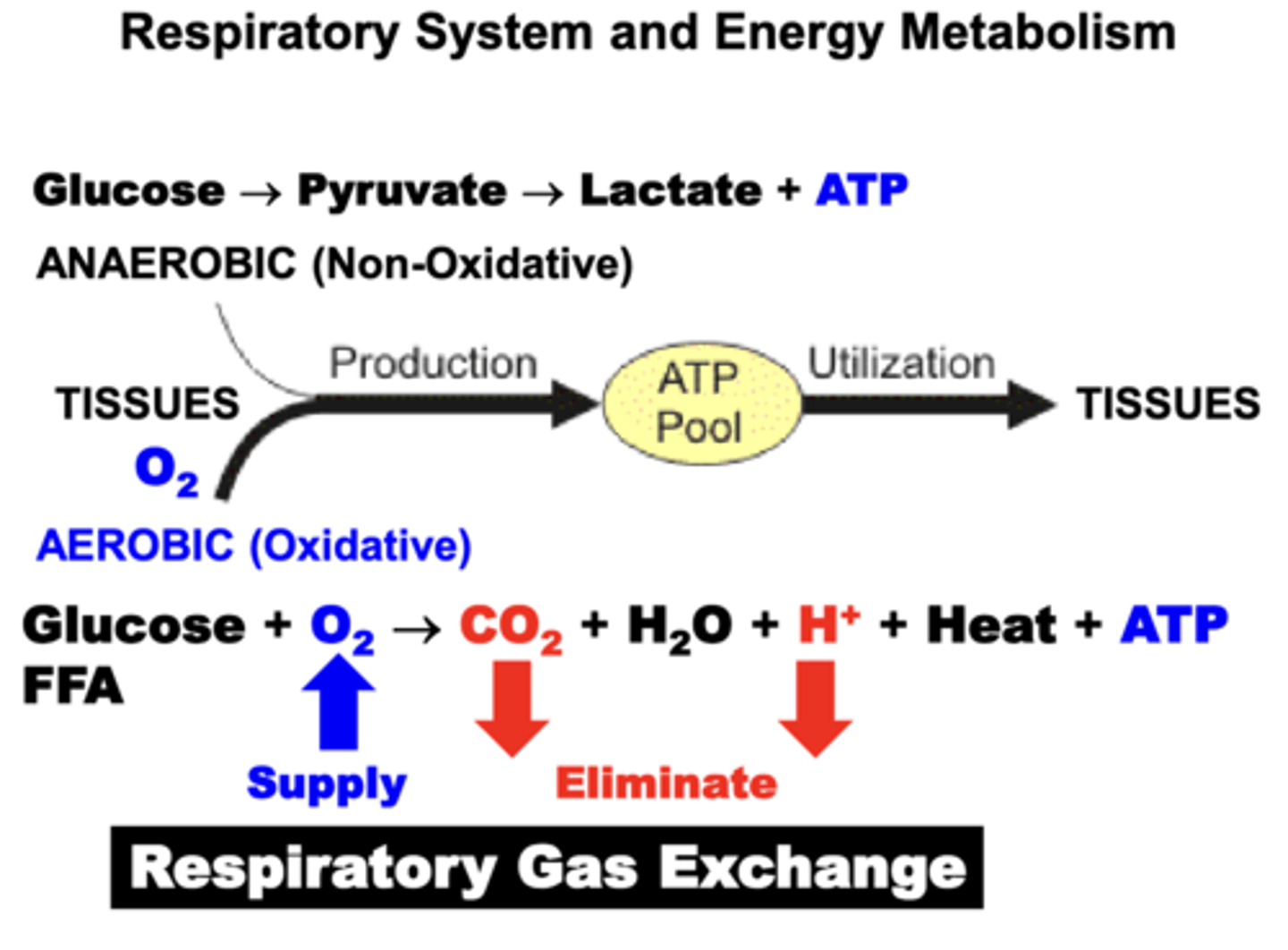
Energy (ATP) requirements of the Tissues are achieved through?
a combination of Anaerobic (Non-Oxidative) and Aerobic (Oxidative) metabolic pathways.
- for most tissues, aerobic production of energy via the Citric Acid (Krebs) Cycle is the major source of energy
- oxidative pathways results in the production of a number of metabolic by-products (H2O, CO2, H+, Heat) that must be eliminated in order to maintain respiratory homeostasis
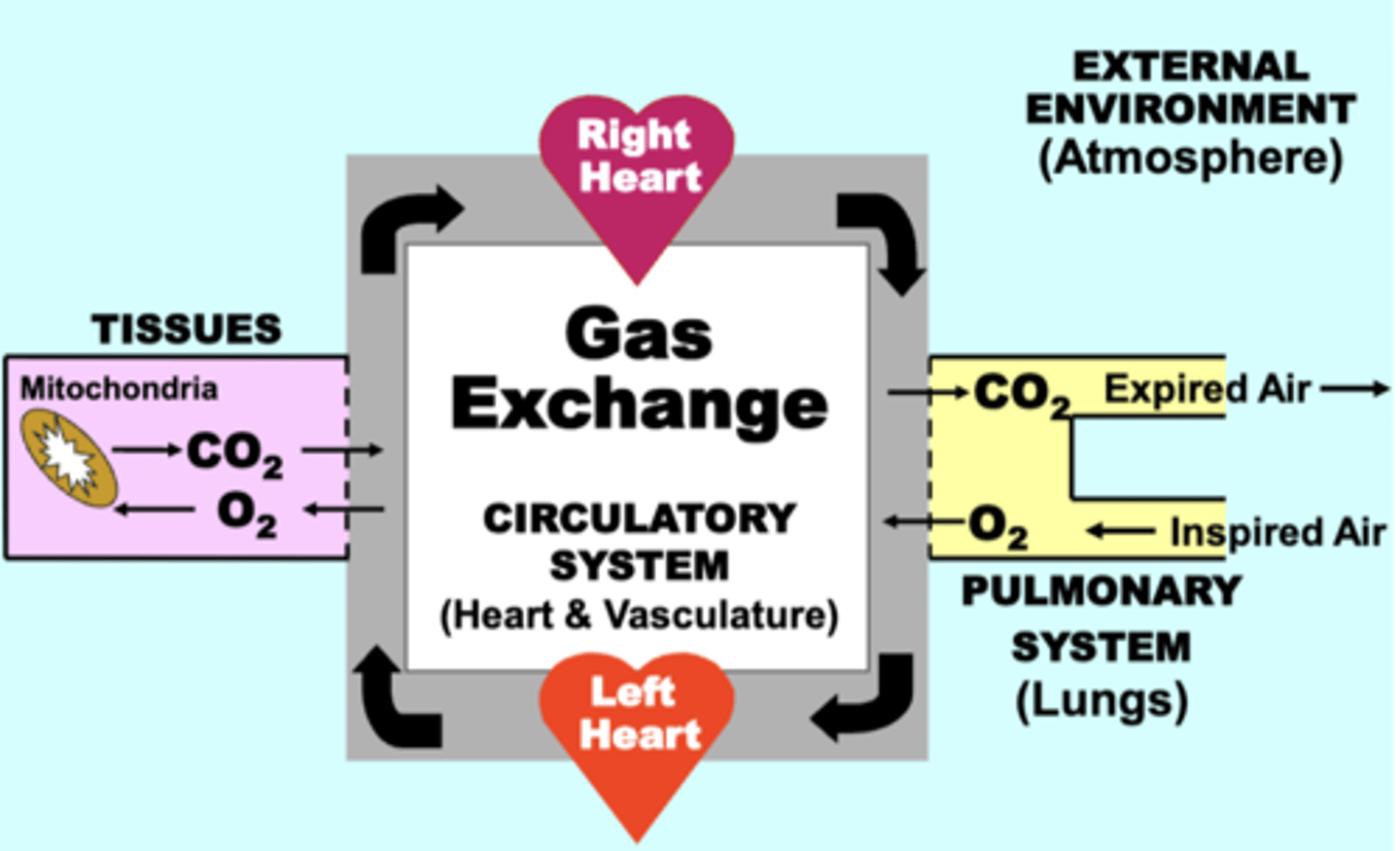
respiratory system circuit
through which the exchange of O2 and CO2 occurs continuously between four basic physiological compartments:
• EXTERNAL ENVIRONMENT (Atmosphere)
• PULMONARY SYSTEM (Lungs)
• CIRCULATORY SYSTEM (Heart & Vasculature)
• TISSUES (Mitochondria)
normal functioning of ALL four compartments is essential for maintaining adequate respiratory function
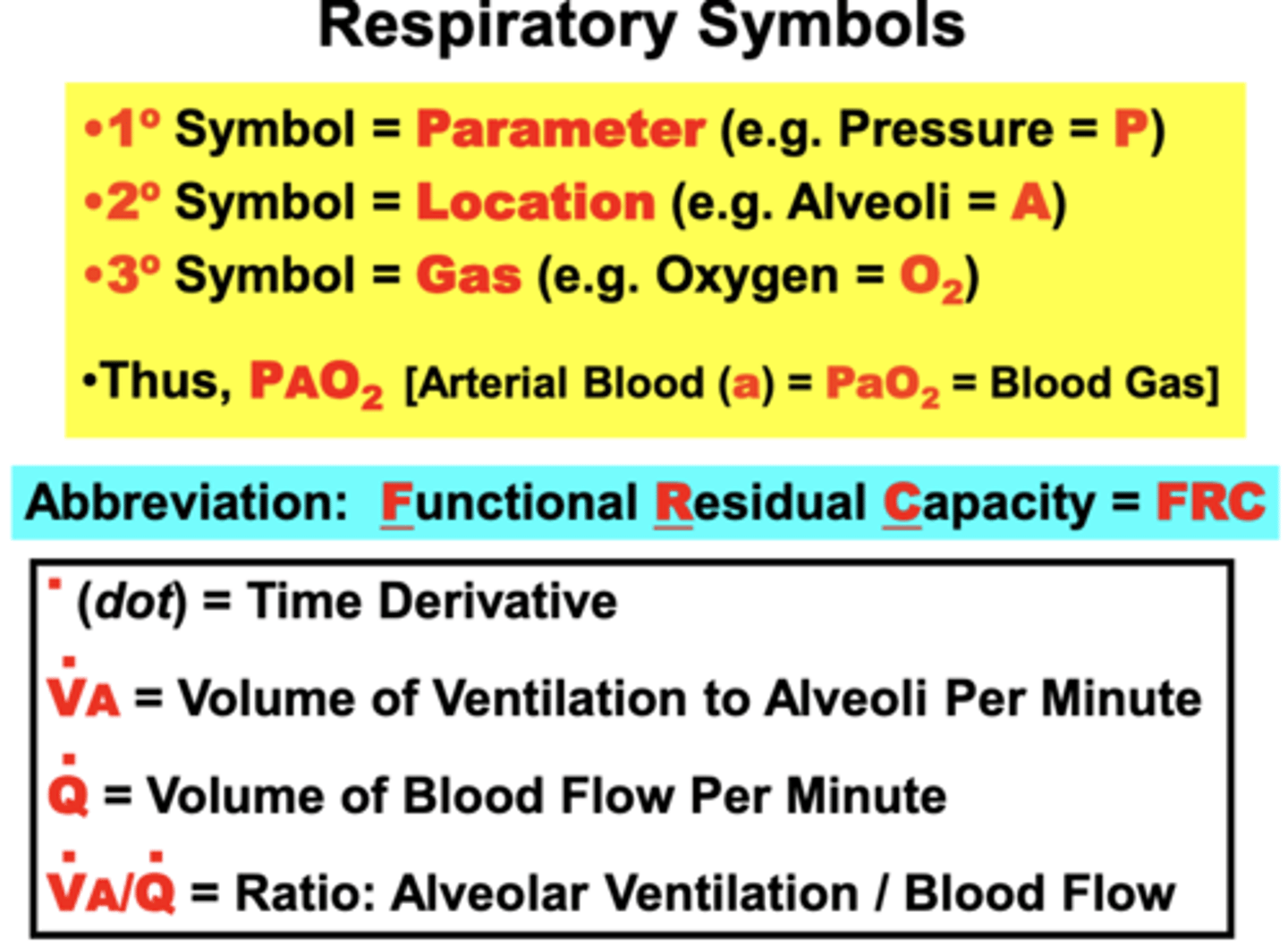
fundamental organization of respiratory symbols
1° symbol = parameter
- ex. Pressure = P
2° symbol = location where the parameter was measured
- ex. Alveoli = A
3° symbol = specific parameter
- ex. particular Gas (oxygen - O2)
- Superscript dot over the primary symbol = time derivative or rate
Disorders that disrupt O2 homeostasis most commonly cause?
hypoxemia, less commonly also cause hyperoxemia

hypoexmia
abnormally low blood O2 level
- Leads to hypoxia, which is abnormally
low tissue O2
- Ultimately impairs internal respiration
Hyperoxemia
abnormally high blood O2 level
- Leads to hyperoxia, which is abnormally high tissue O2
- Can cause fatal damage to tissues (ROS) if sustained long periods
Disorders that retain excessive CO2 in the body cause?
Hypercapnia

Hypercapnia
abnormally high blood CO2 level
- Leads to acidemia (acidosis), which is abnormally low blood pH
- body is retaining excessive CO2
AKA - hypercarbia
excessive elimination of CO2 from the body causes?
hypocapnia

Hypocapnia
abnormally low blood CO2 level
- Leads to alkalemia (alkalosis), which is abnormally high blood pH.
- Body is excessively eliminating CO2
AKA - Hypocarbia
Gas Partial Pressure
pressure exerted by individual gas in mixture of different gases
- Pgas
- dependent upon the total gas pressure (Ptotal) and the Fgas
= Ptotal x Fgas
Gas Fractional Concentration
percentage of individual gas in mixture of different gases
- expressed as the percentage (%) of each individual gas in a mixture and is abbreviated as (Fgas)
- total must ALWAYS by 100%
Gas Volume
volume occupied by individual gas in a non-fluid environment
Gas Content
amount of an individual gas in a fluid environment
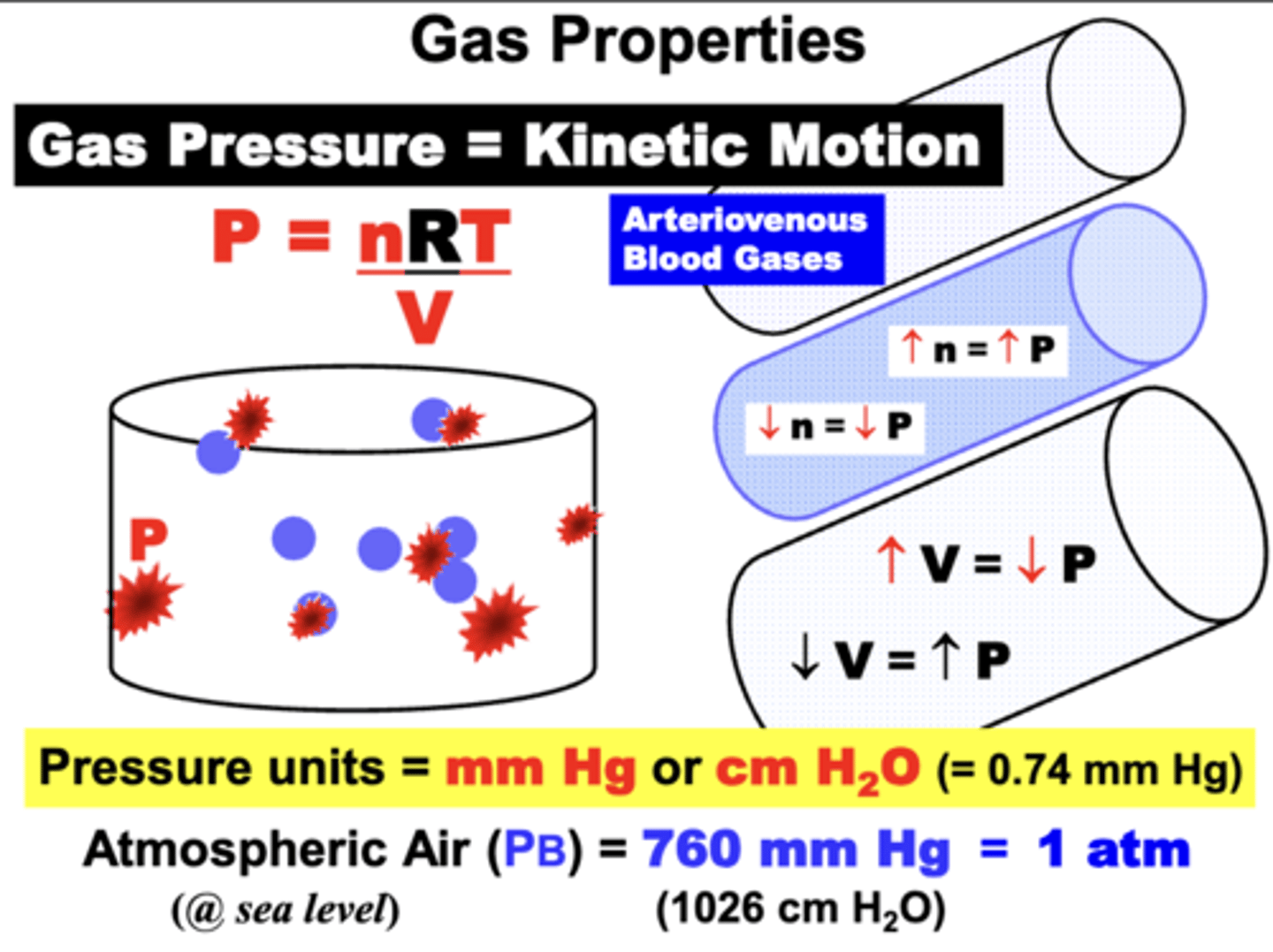
gas pressure results from?
the Kinetic Motion of gas molecules colliding against each other and the walls of a container (e.g. a blood vessel wall or alveolar membrane)
pressure units
1. mmHg
- commonly called Torr (1 mmHg)
- = 1.35 cmH2O
2. cmH2O
- =0.74 mmHg
- smaller pressures
Pressure caused by the kinetic motion of gases in the blood and vasculature can be quantified with?
specific gas-sensing electrodes
- These gas pressures are the parameters that are measured when clinical Arteriovenous Blood Gas samples are determined from patients
ideal gas equation parameters
P = nRT/V
1. Number of molecules (n = moles of gas)
2. Temperature (T = 273° K @STPD)
- Dry Air (STPD)
3. Container Volume (V = Liters)
4. Gas Constant (R = 62.36 L mmHg K-1 mol-1 )
Atmospheric Air at sea level under ideal dry ambient conditions
ATM
- exerts 760 mmHg at sea level (1 ATM)
- commonly referred to as Barometric or Atmospheric pressure (PB)
mixture:
- N2 (78%)
- O2 (21%)
- CO2 (21%)
- other <0.87%
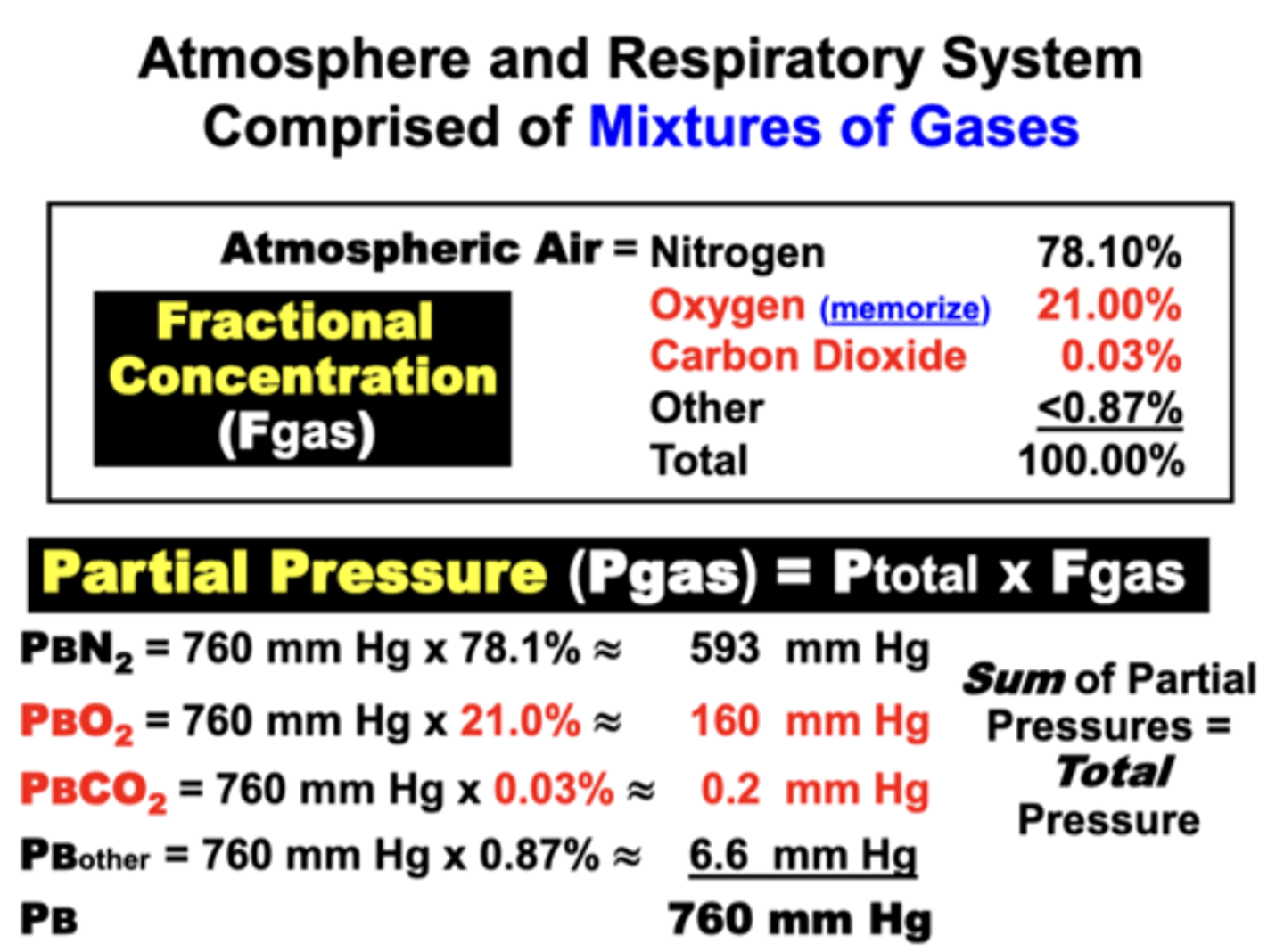
the sum of all individual gas partial pressures must always be equal to?
the Ptotal of the mixture under a given set of conditions (e.g. 760 mm Hg at sea level)
PB/FB Values
**MEMORIZE**
PB = 760 mmHg
PBO2 = 160 mmHg
PBCO2 = <1 mmHg
FBO2 = 21%
FBCO2 = <0.03%
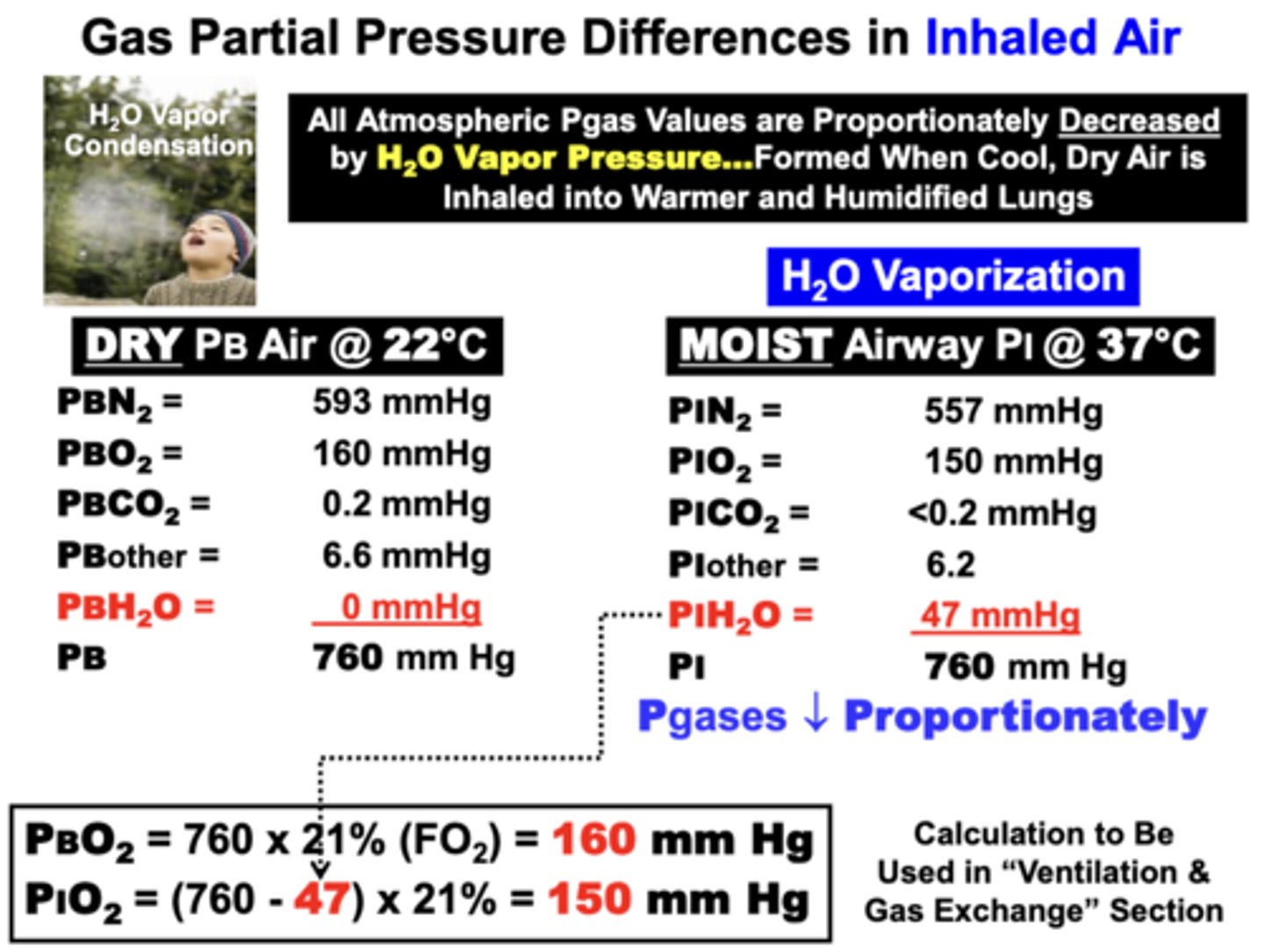
Gas Partial Pressure Differences in Inhaled Air
All Atmospheric Pgas values are proportionately decreased by H2O Vapor Pressure
- formed when cool, dry air is inhaled into warmer and humidified lungs
When a volume of dry, ambient temperature (= 22°C) atmospheric air is inhaled into the moist & warmer temperature airways of the lungs → all component Pgas values decrease proportionately while total gas pressure remains 760 mm Hg
why are all Atmospheric Pgas values are proportionately decreased by H2O Vapor Pressure?
because the PH2O of dry ambient temperature air is 0 mm Hg
- once inhaled, PH2O increases to 47 mm Hg as air is humidified upon entering the moist respiratory passageways at the warmer body temperature (37°C)
- Higher temperature vaporizes H2O fluid into
gas form
= Since total gas pressure in the system must remain constant at any given altitude (760), available pressure for other component gases must proportionately decrease
PBO2 = 160 mmHg
PIO2 = 150 mmHg
what are the four respiratory compartments?
1. pulmonary capillaries
2. arterial
3. tissue capillaries
4. venous
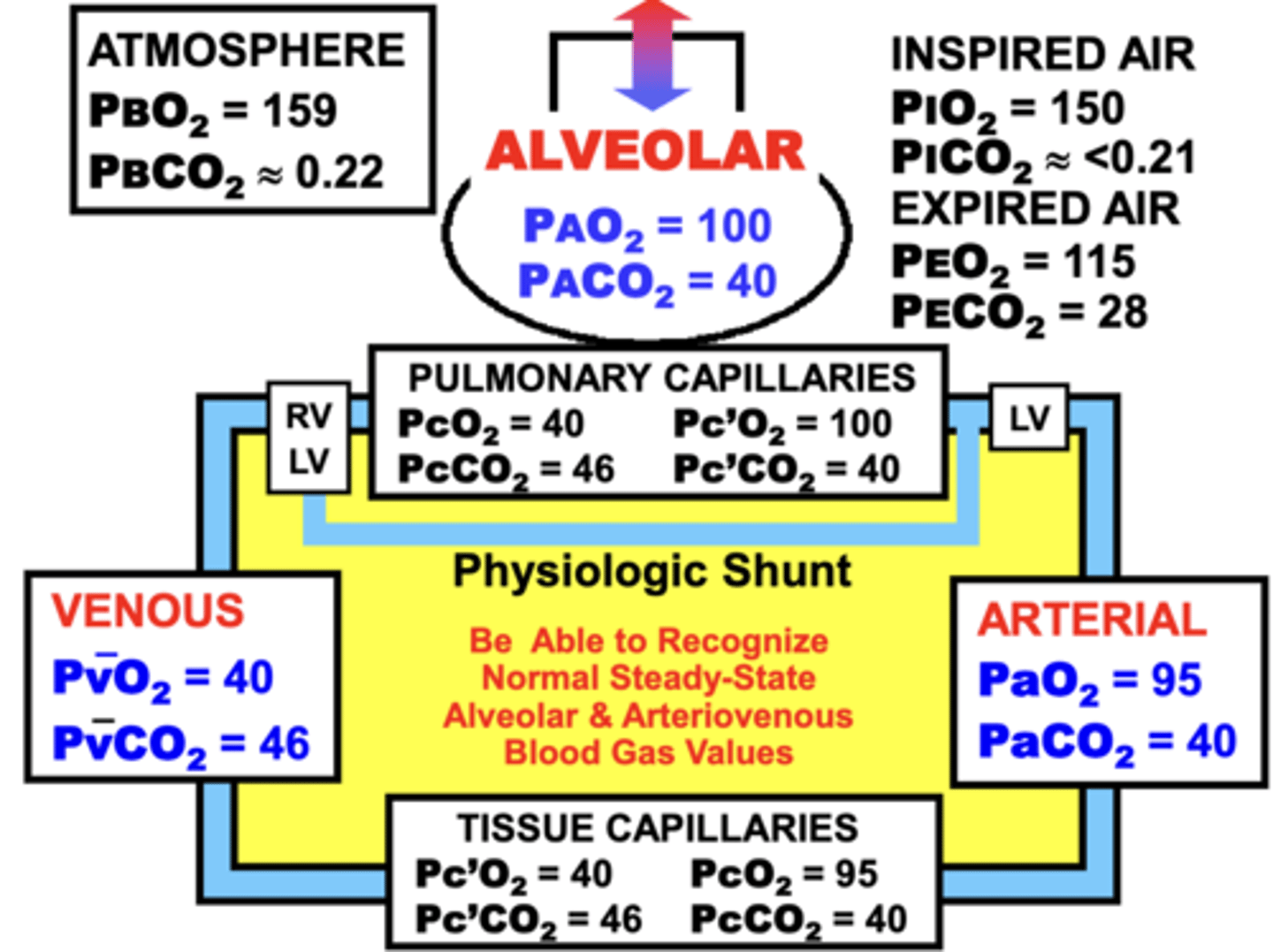
Maintaining the steady-state partial pressure values in the respiratory compartments is important to?
1. Maintain appropriate gas concentrations in blood and tissues.
- When steady-state values abnormally increase or decrease, gas concentrations are affected in parallel
- results in excessive or deficient O2 and/or CO2 levels in the various respiratory compartments
2. Maintain gas movement across the respiratory system
- Gas movement across certain respiratory compartments is dependent on gas gradients between compartments
gas exchange between the respiratory compartments
- occurs by gas movement or transport between the four respiratory compartments
- transport is complicated by the presence of biological membrane barriers between some. compartments
two basic mechanisms for gas movement
1. bulk flow
2. passive diffusion
bulk flow
pulmonary system:
- moves O2 and CO2 between the pulmonary airways and the external environment by bulk flow of alveolar and atmospheric air into and out of the lungs
cardiovascular System
- also moves gases between the lungs and tissues by bulk flow in arterial and venous blood
- clinical disorders which impair pulmonary or CV function cause disruption bulk flow transport mechanisms
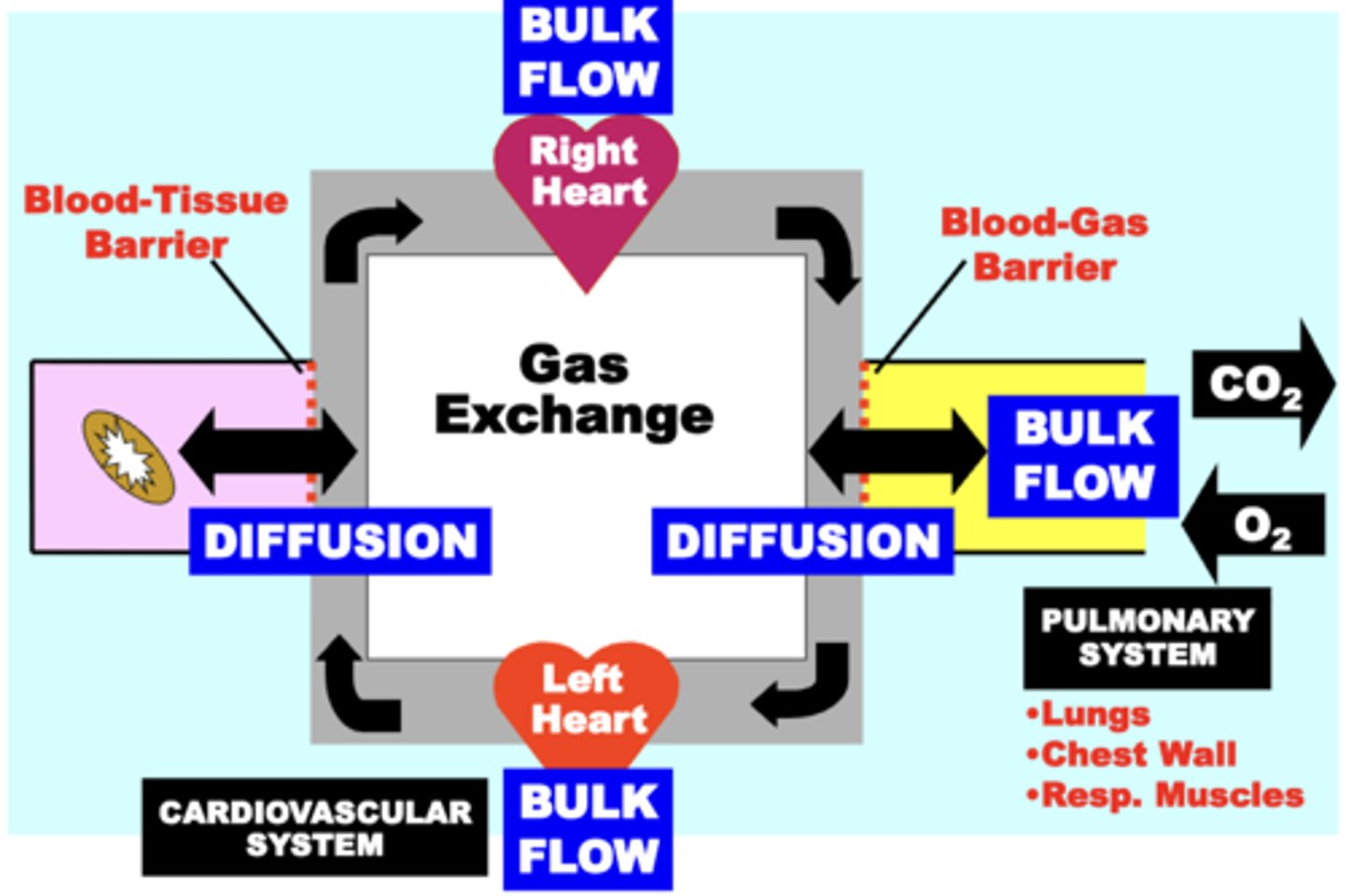
Passive Diffusion
transports gases across biological membrane barriers within the respiratory circuit
- provides a physiologically important and energetically favorable mechanism for the movement of these gases within the respiratory system
- disorders that damage membrane barriers (e.g. emphysema) pathophysiologically impair gas diffusion
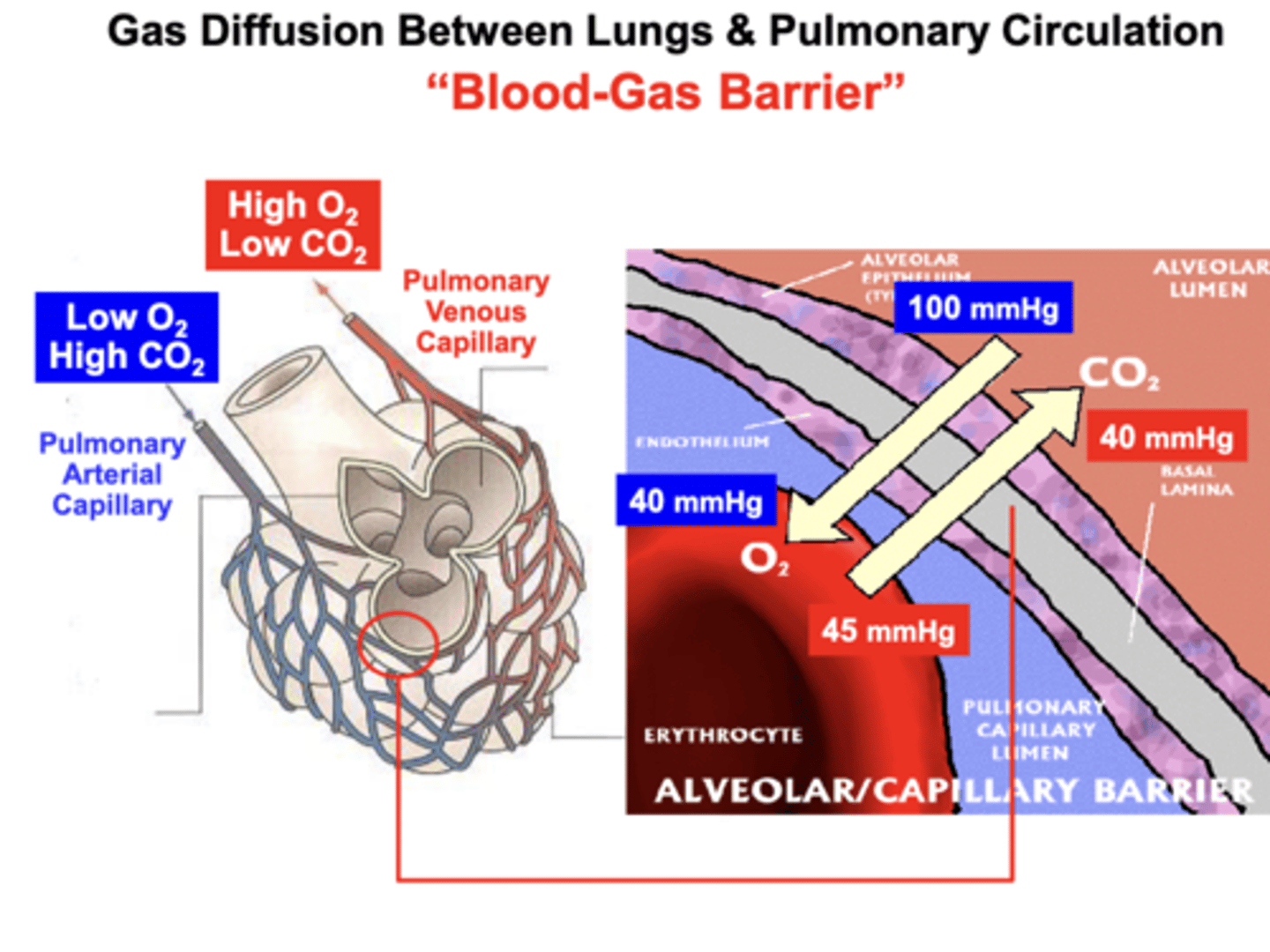
Blood-gas barrier
between the alveolar and pulmonary capillary membranes
- AKA the Alveolar-Capillary Barrier
O2: moves across the barrier down a passive Diffusion Gradient
- from the Alveolus into the Capillary blood (erythrocyte) to replenish or “Re-Saturate" the blood with O2
CO2: moves across the barrier down a passive Diffusion Gradient
- from the Capillary into the Alveolus, which is then exhaled from the lungs into the atmosphere to eliminate excess CO2 from the body
Blood-tissue barrier
between arteriovenous capillary and tissue cell membranes
Passive Diffusion of gases across respiratory membranes is dependent upon?
the development and maintenance of Steady-State Gas Pressure Gradients between compartments
direction & volume of flow of a particular gas is determined by the?
steady-state gas pressure difference between compartments
O2 pressure gradient
drives O2 out of the lung and into pulmonary arterial blood (blood-gas barrier)
- then drives O2 out of systemic arterial blood and into tissues and eventually into mitochondria (blood-tissue barrier) for oxidation
Net direction of flow of O2: lungs → tissues
CO2 pressure gradients
drive CO2 out of the tissues and into systemic arterial blood
- then drives CO2 into the lungs (alveolar gas) and out of pulmonary arterial blood
Net direction of flow of CO2: tissues → lungs
disruption or reducing of steady state pressure gradients can decrease?
gas diffusion & gas exchange, potentially resulting in Hypoxemia &/or Hypercapnia
Decreasing Steady-State Gas Pressure Gradients between respiratory compartments decreases?
gas diffusion volume and rate between compartments
Emphysema
- pulmonary disorder
- ultimately causes Hypoxia
disrupts (decreases): the gas pressure gradient between the lung compartment (Alveolar Gas) and pulmonary blood compartment (Pulmonary Arterial Blood)
causes: less O2 is replaced in the blood, which decreases the O2 supply to the tissues leading to hypoxemia and tissue hypoxia
increasing Steady-State Gas Pressure Gradients between respiratory compartments increases?
gas diffusion between compartments, which can restore normal O2 supply to tissues
clinical treatment of emphysema
high FO2 air (100 O2 Air) is an effective treatment for Hypoxia
- increasing the gas pressure gradient between the lung compartment (Alveolar Gas...800 mmHg) and pulmonary blood compartment (Pulmonary Arterial Blood...40 mmHg) restores Arterial Blood PaO2 to near a normal level (90mmHg) such that O2 Supply is Restored to the tissues (Tissue Normoxia, 38mmg)
Avogadro's Law
equal volumes of all gases, at the same temperature and pressure, have the same number of molecules
- for a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant
= 1 Mole of Any Gas Occupies a Volume of 22.4 L at STPD
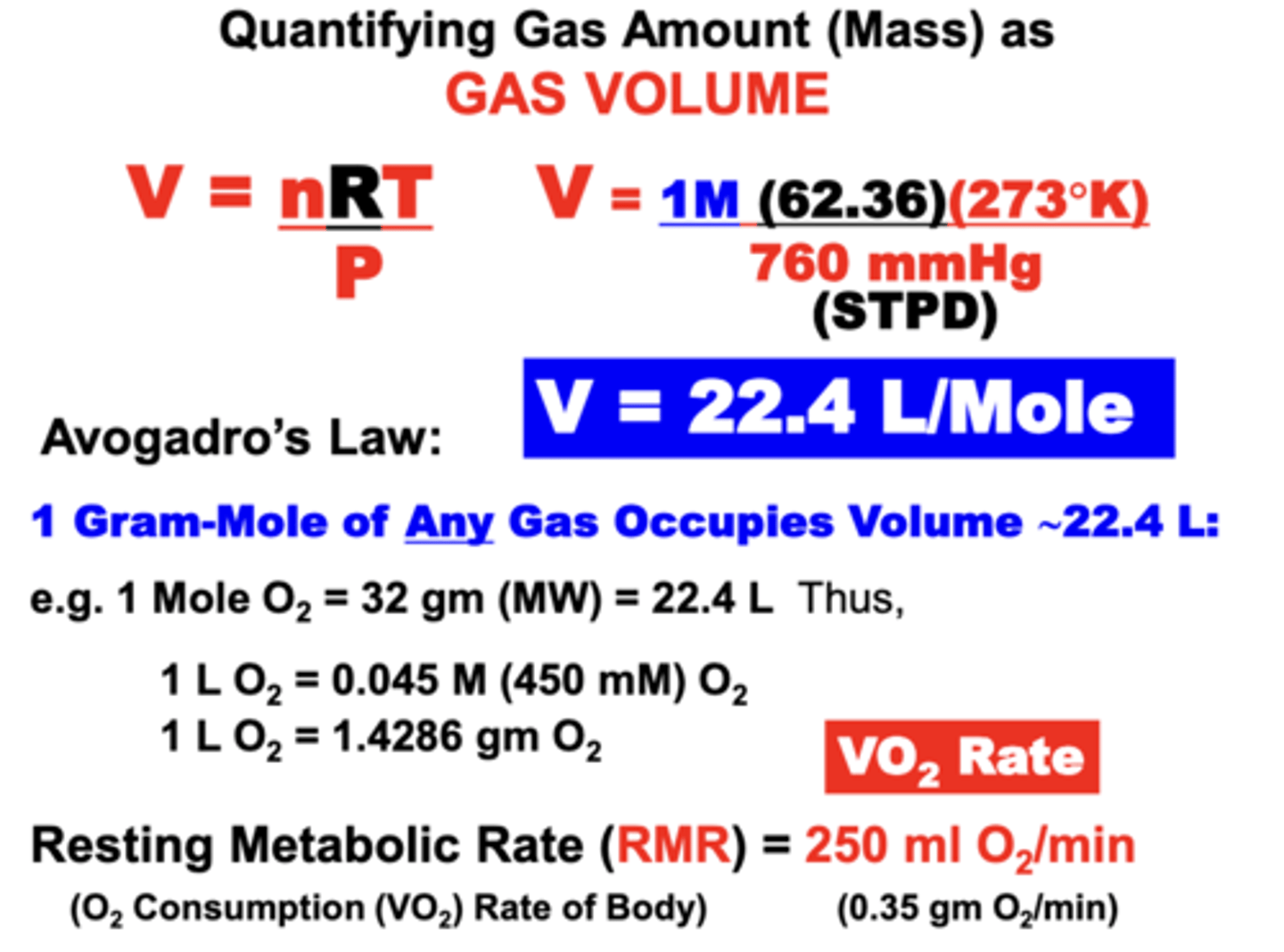
normal Resting Metabolic Rate
RMR
- of a 70kg male = 250 ml O2/min (0.35 g O2/min)
- more commonly expressed as O2 Consumption Rate (VO2 rate)
When gases move from a non-fluid compartment (i.e. alveolus) to a fluid medium (i.e. plasma), a
proportion of gas is?
solubilized (dissolved) in the fluid through interactions w/ H2O
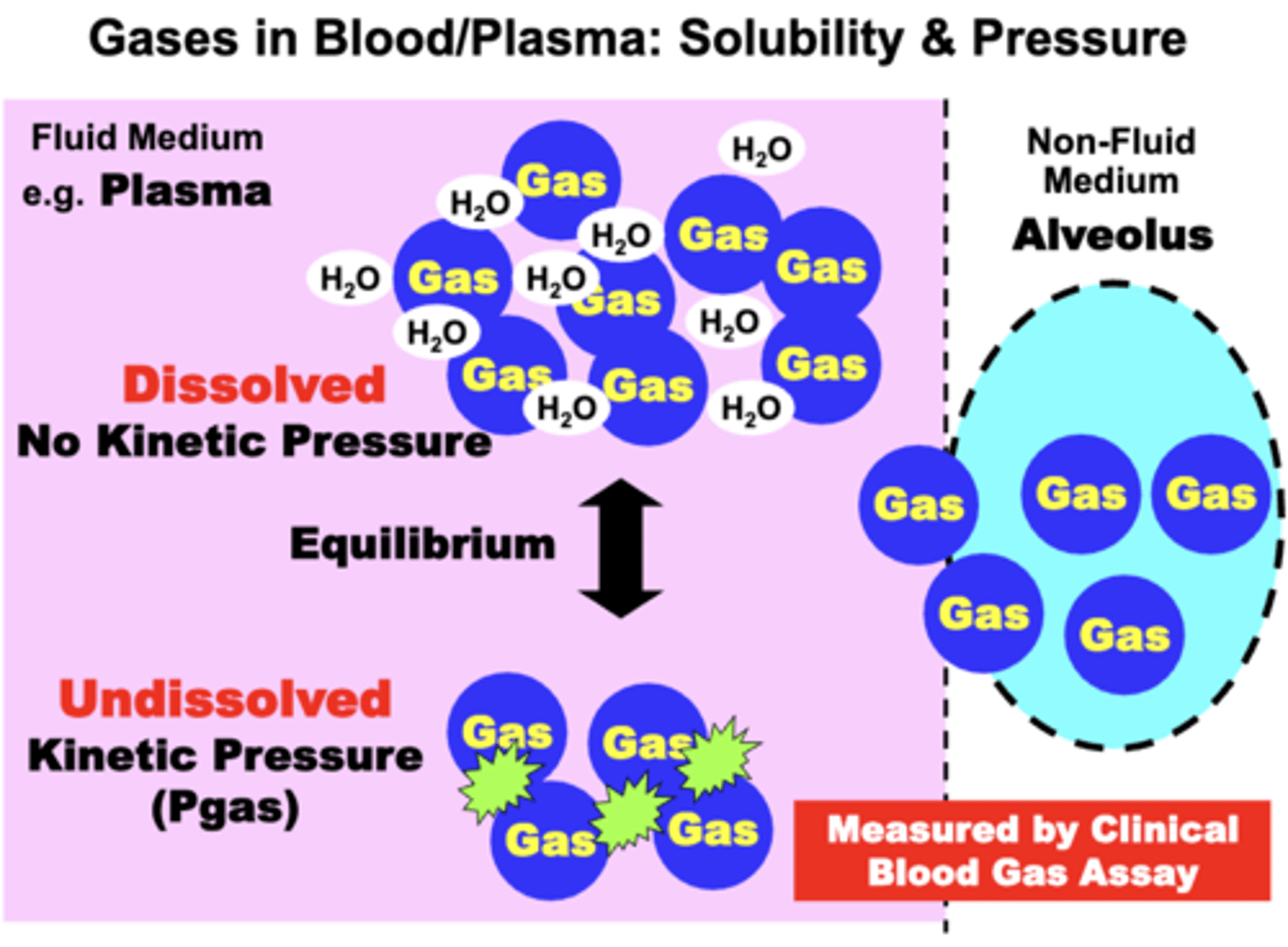
Dissolved gas molecules DO NOT contribute to ___ ___ because?
gas pressure; they DO NOT generate kinetic pressure in the medium
Dissolved gas molecules DO contribute to?
the total amount of gas (vol%) in the medium (i.e. Total Gas Concentration)
Most gases solubility is ____ in biological fluids, meaning?
limited; some gas molecules entering plasma remain undissolved and behave as if in a non-fluid compartment
importance of the undissolved gas fraction
- insignificant in quantity relative to the dissolved fraction
- BUT, is important because it exerts the Kinetic Pressure (Pgas) that is measured by specific gas sensing electrodes that are utilized for clinical Blood Gas assays
Dissolved and undissolved gases exist in an?
equilibrium flux
- gas pressure exerted by the undissolved gas fraction is proportional to the dissolved gas concentration
quantification of dissolved gas concentration
quantified based on empirical measurement of gas pressure
▪ Calculation of dissolved gas is described in terms of Henry’s Law
Respiratory gas volumes in blood are quantified in terms of its?
Gas Content
- e.g. O2 Content in blood
gas content
conventional term used to express Gas Concentration
- standard units are ml gas/100 ml of blood volume
- shorthand for this unit is volume percent (vol%)
- average normal O2 content in arterial blood is 20 ml O2/100 ml blood → 20 vol%
Each gas in a mixture differs in its ability to?
solubilize in fluids
relative solubility
expressed in terms of a solubility coefficient
- unique for each gas
- based on its chemical and physical properties and the nature of the medium (i.e. plasma vs. water)
Dissolved gas content of a particular gas in plasma is proportional to the?
product of its specific solubility coefficient and its empirically measured partial pressure (Pgas) in the plasma
- expressed by Henry's Law
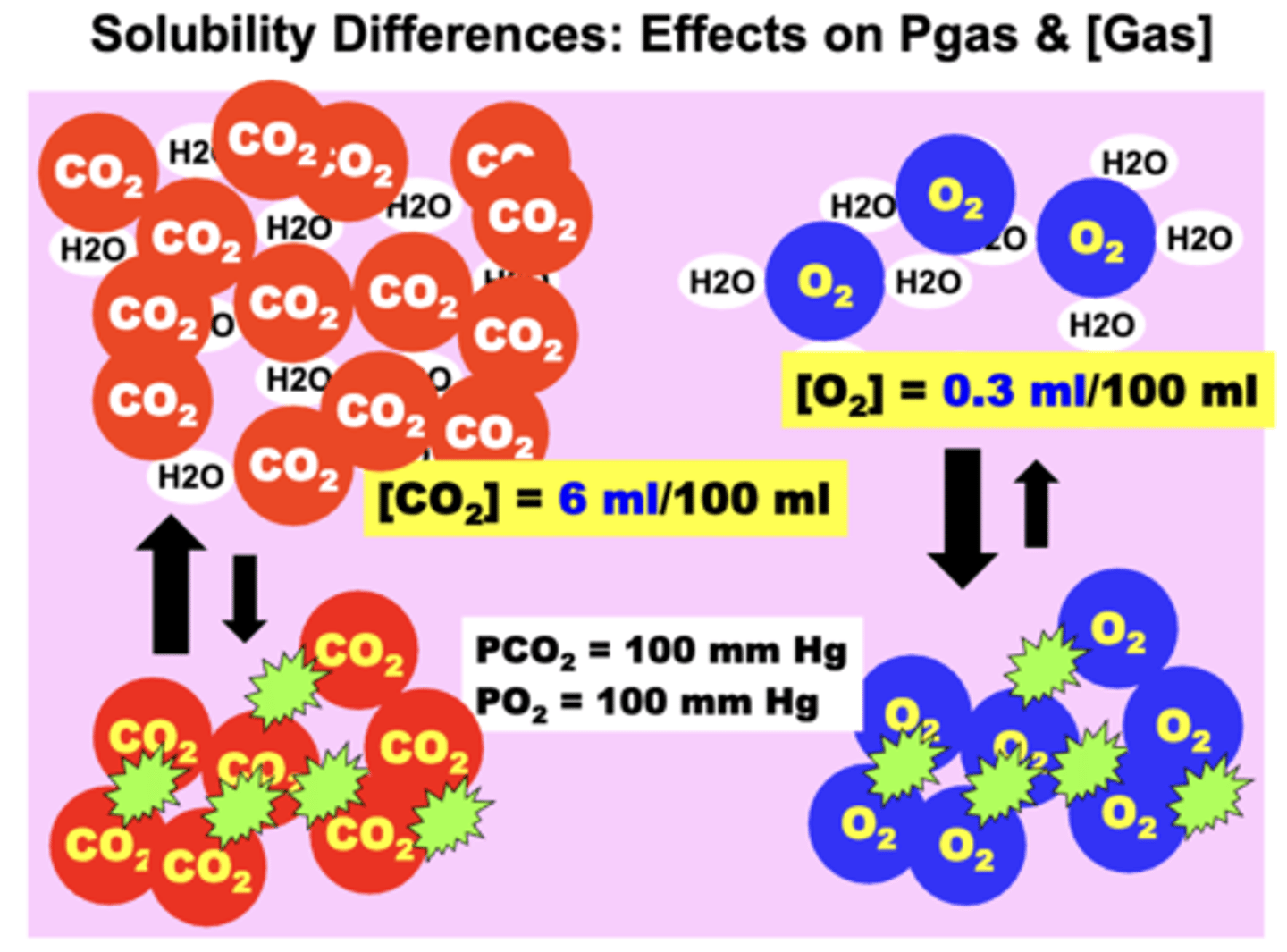
NOTE: the dissolved content of gases that differ in plasma solubility can be quite different at the same partial pressure and vice versa
- ex. CO2 is 20x more soluble in plasma compared to O2
units of [gas]
ml gas/100 ml fluid
Solubility of CO2 compared to O2
CO2 is 20x more soluble in plasma compared to O2
- much more dissolved CO2 in the plasma may exist than O2 even though the PO2 value may be much higher than the PCO2 value
o If the two gases are present at the SAME partial pressure (PCO2 = PO2) → O2 will possess a lower vol% than CO2
o If the two gases are present at the SAME concentration (O2 vol% = CO2 vol%) → O2 will exert a higher Pgas than CO2
Greater gas solubility facilitates a greater?
gas diffusion across biological membranes
- Higher solubility gases diffuse more easily across membrane barriers than lower solubility gases
- ex. CO2 is 20x more soluble than O2
- 20x more CO2 than O2 can diffuse across the blood-gas barrier at the same partial pressure gradient (if only solubility properties are considered)
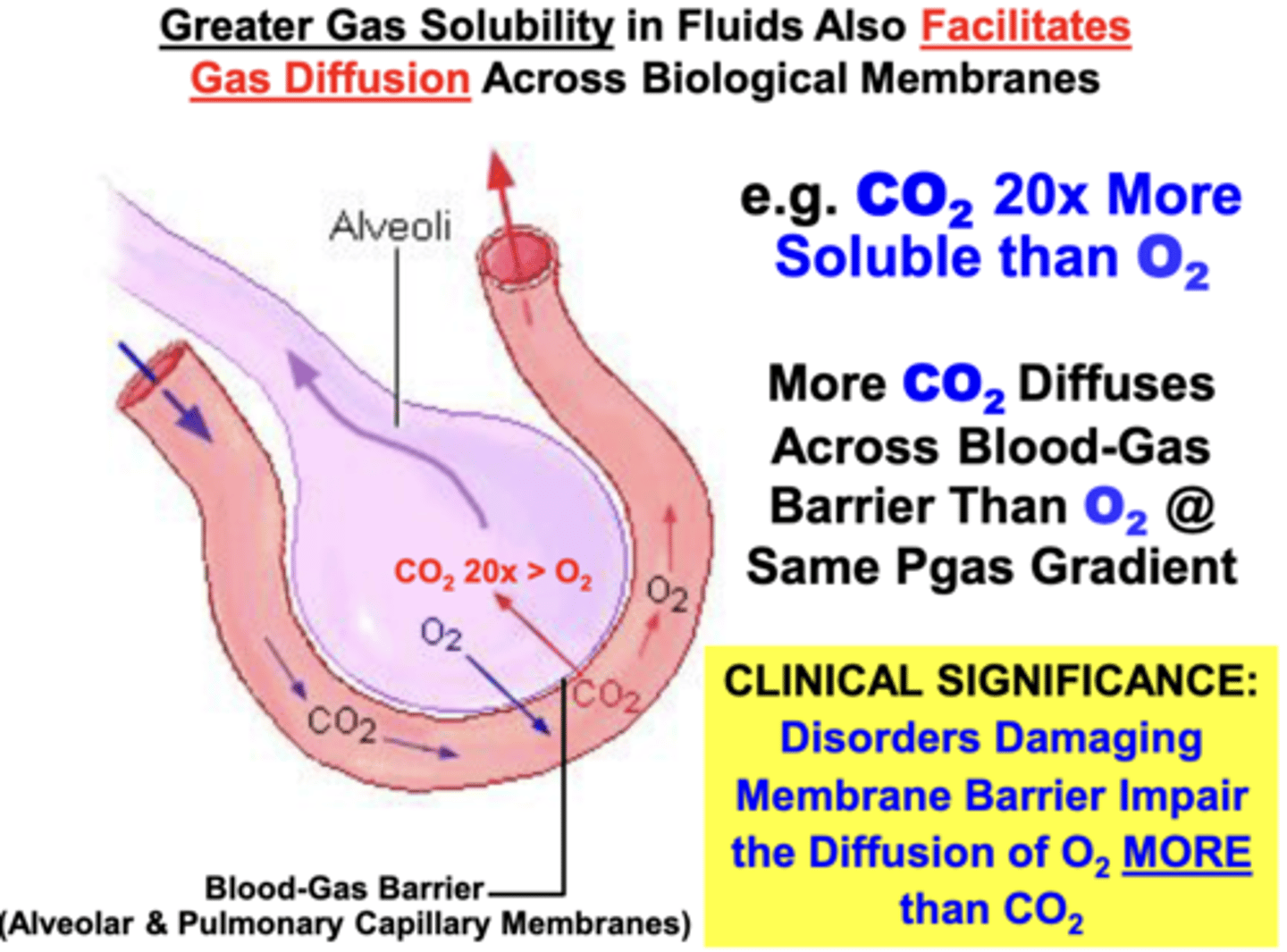
clinical significance of the solubility of CO2 compared to O2
disorders damaging the integrity of the membrane barriers (i.e. inflammatory fibrosis → thickens barrier) impair diffusion of O2 more than CO2
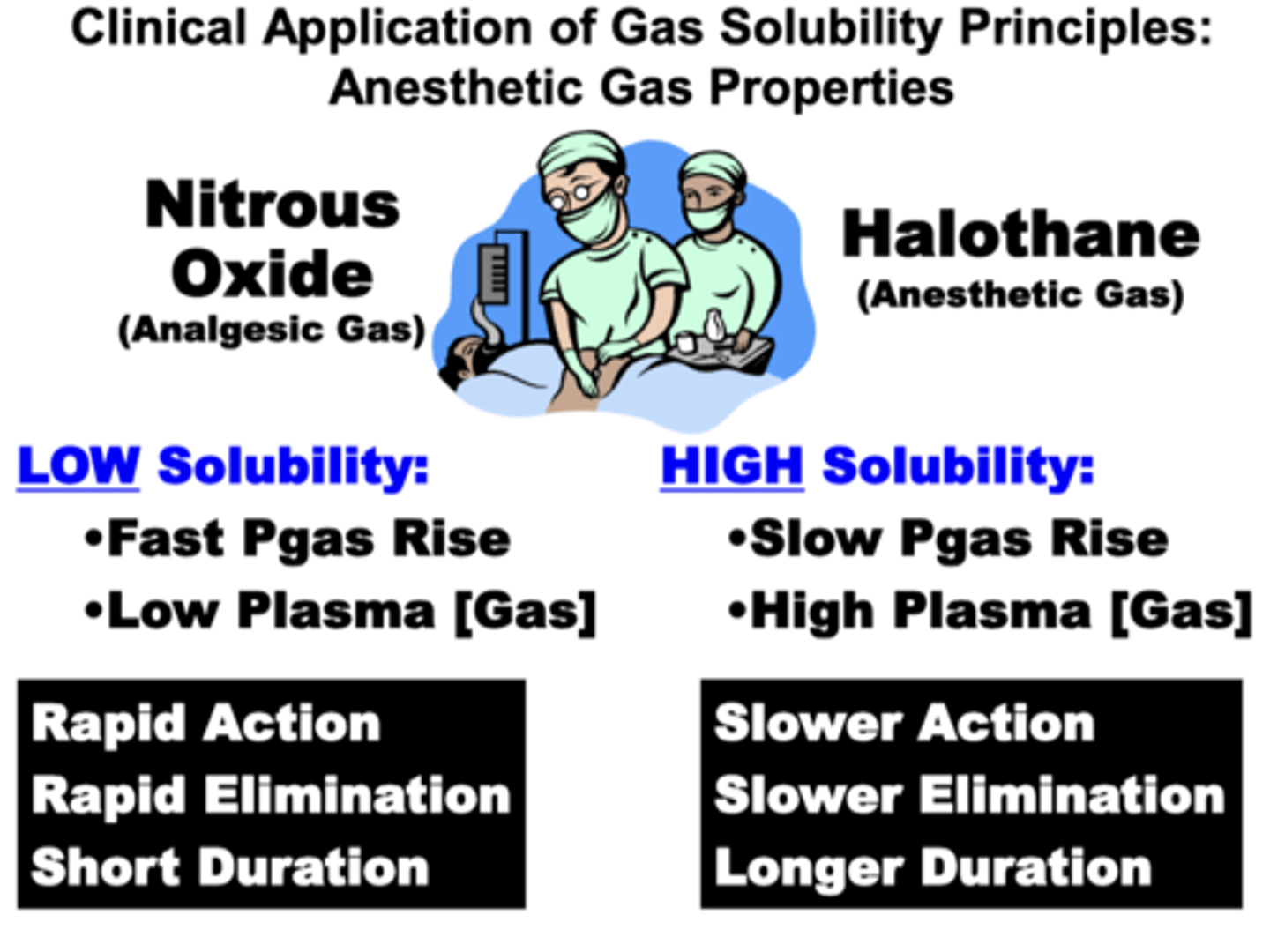
Clinical Application of Gas Solubility Principles: Anesthetic Gas Properties
Halothane (anesthetic gas) vs. Nitrous Oxide (analgesic gas)
- differ in their relative solubility in plasma
Nitrous oxide:
→ low solubility
→ increased amount of undissolved gas
→ fast Pgas rise
→ low plasma vol%
→ rapid action, rapid elimination, short duration
Halothane:
→ high solubility
→ increased amount of dissolved gas
→ slow Pgas rise
→ high plasma vol%
→ slower action, slower elimination, longer duration
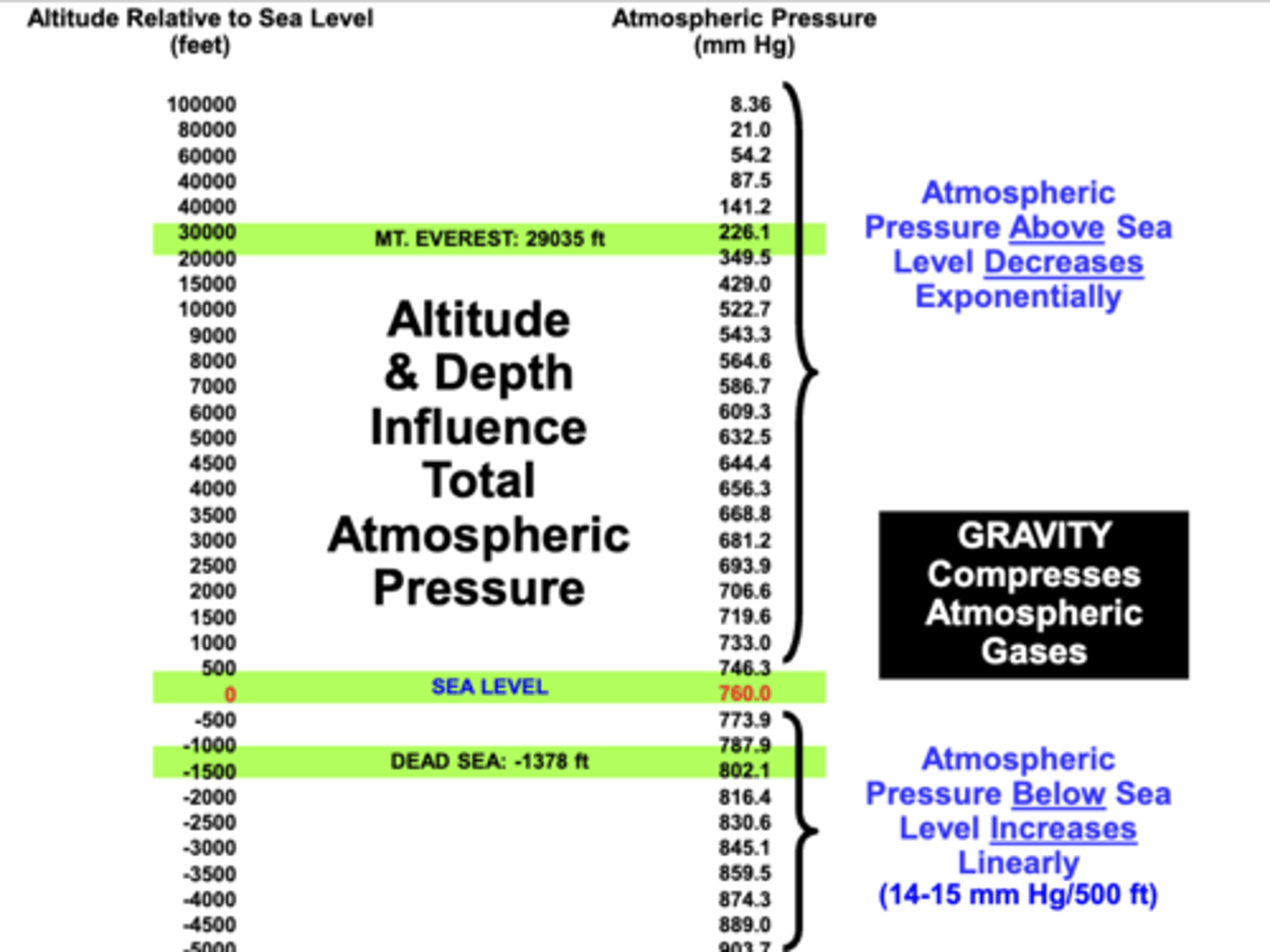
Gravity's effect on atmospheric air gases
compressing the gases in atmospheric air such that the total atmospheric air pressure (PB):
1. decreases during ascent to altitudes above sea level
2. increases during descent to depths below sea level
o Atmospheric pressure above sea level → decreases exponentially
o Atmospheric pressure below sea level → increases linearly (14 – 15 mm Hg/500 ft)
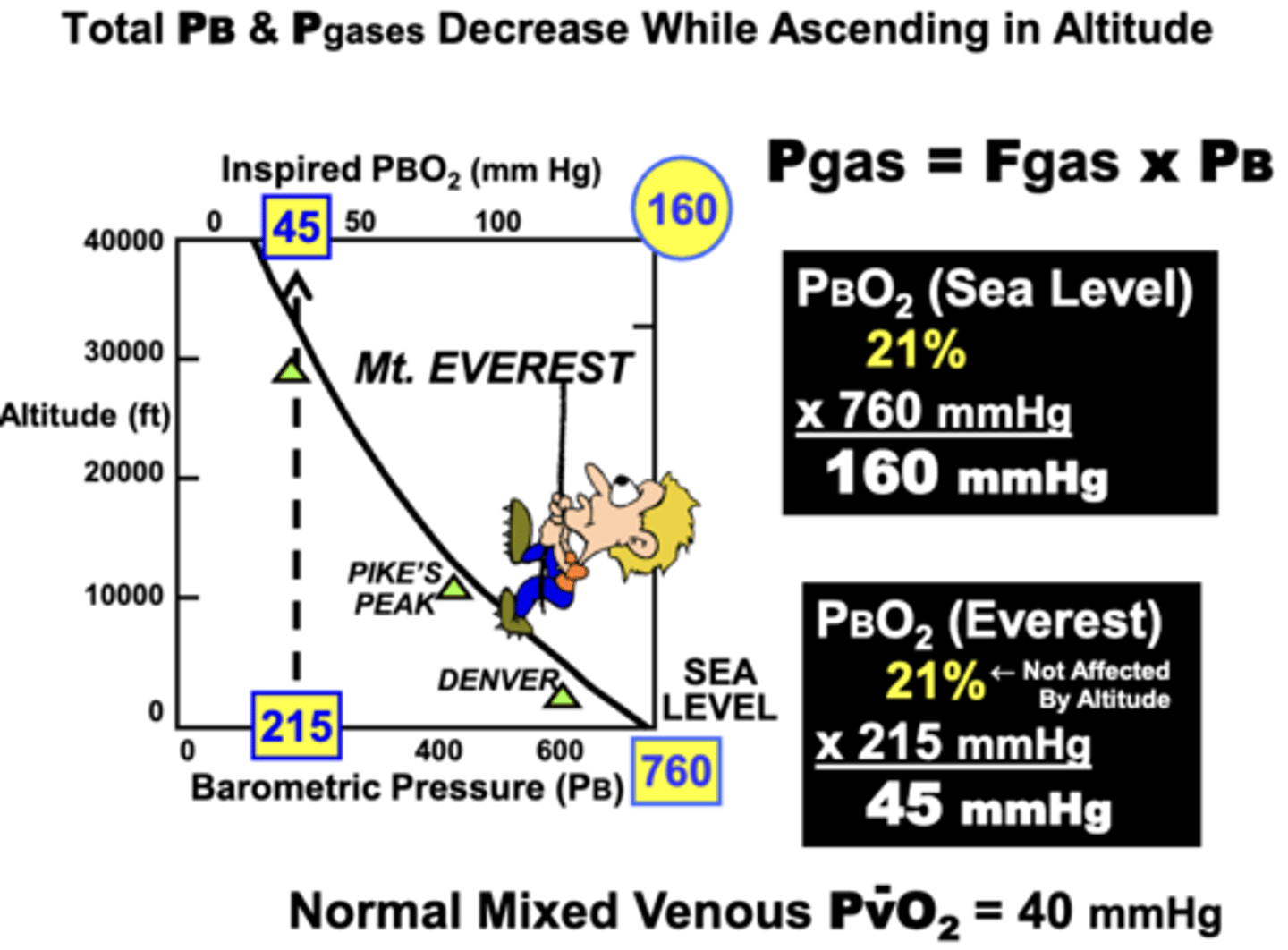
change in total PB, Pgases, and Fgas when ascending in altitude
Total PB and Pgases: decrease while ascending
Fgas: remains the unaffected by altitude
ex.
▪ At sea level → 760 mm Hg (PB) x 21% (FO2) = 160 mm Hg (PBO2)
▪ At Mt. Everest → 215 mm Hg (PB) x 21% (FO2) = 45 mm Hg (PBO2) (near PvO2 at sea level)
• This decrease or ‘thinning’ of PB and inspired PO2 largely account for the hypoxemia and breathing difficulties at high altitudes → causes the need for supplemental O2 at very high altitudes
Total body gas pressure equilibrates with?
the total atmospheric pressure surrounding the body
o At sea level, PB = 760 mm Hg → total gas pressure in most compartments in the body is about 760 mm Hg
▪ If PB changes → the total body gas pressure will eventually re-equilibrate to the new surrounding gas pressure value
o However, the partial pressures of various gases are different in the body compared to atmospheric pressure
▪ This is because the body metabolizes O2 during energy production and produces CO2 as a waste product
change in total atmospheric pressure during descent to depths below sea level
increases during descent to depths below sea level
- PB and all Pgases become proportionately affected
- can cause decompression sickness (the ‘bends)
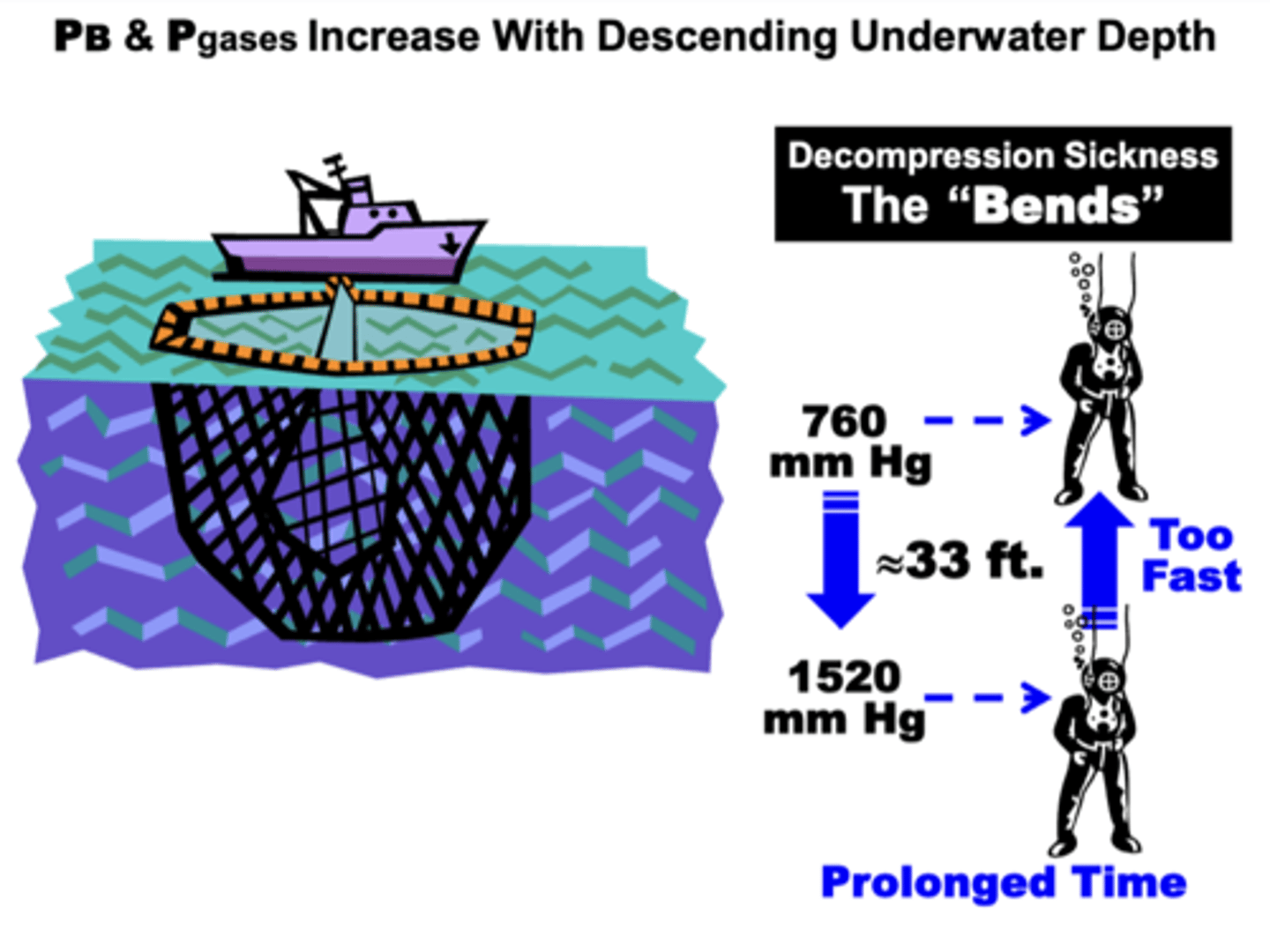
Decompression Sickness
AKA: the Bends, Caissons Disease, Dysbarism
results from:
- the hyperbaric conditions of descending below sea level due to two factors:
▪ Remaining at sub-sea level depths for a prolonged time
▪ Ascending too fast to the surface
related to:
- to difficulties in nitrogen (N2) re-equilibration between the tissue and blood compartments during rapid ascent from depths
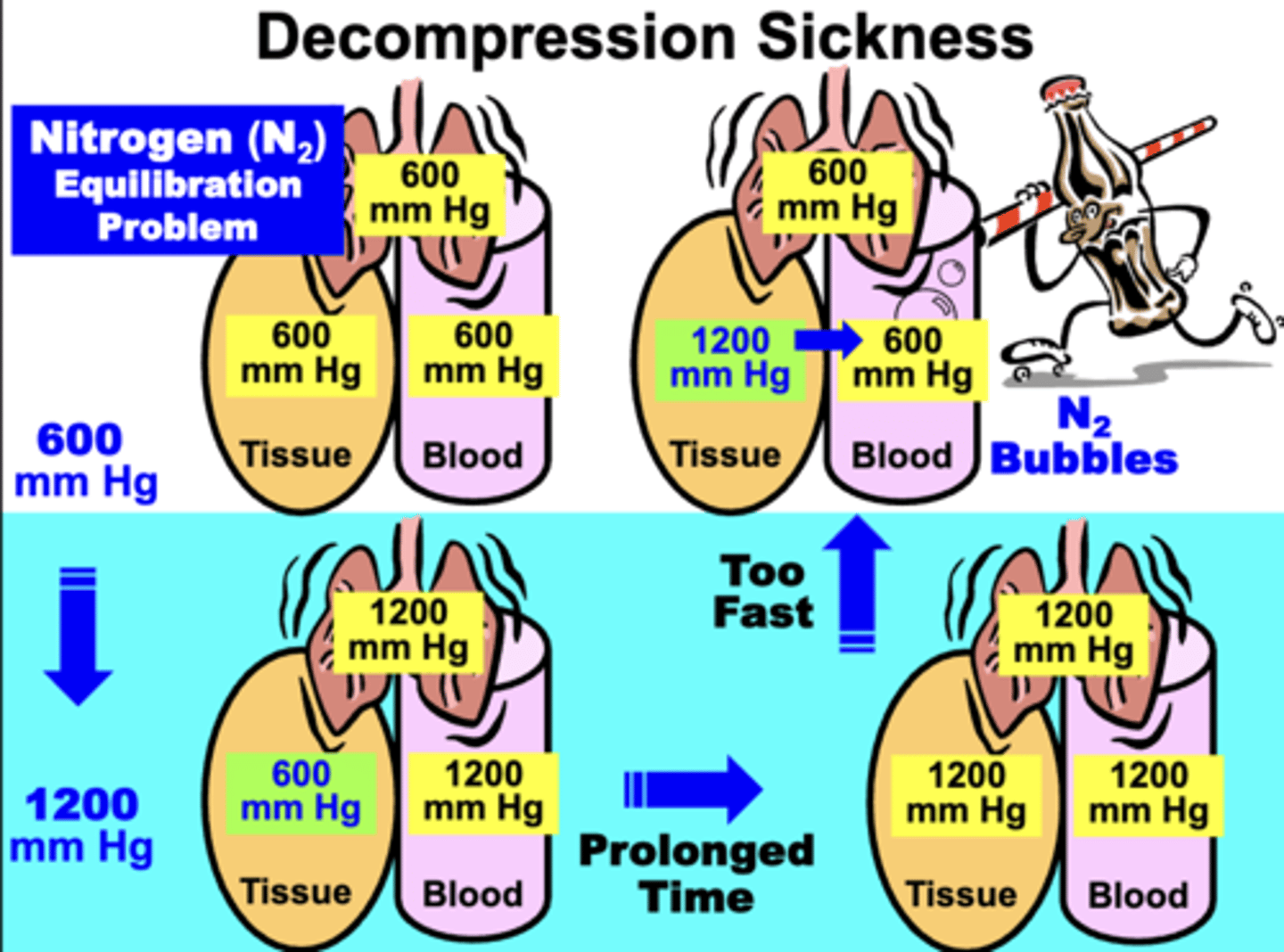
As a diver descends, what occurs?
the increased total PB equilibrates rapidly with pulmonary and blood compartments, while the tissues equilibrate much slower (especially N2 component)
- If diver stays at said depth for only a short time (before tissues equilibrate with N2) → ascending to sea level does not result in the Bends
If diver remains descended for a prolonged time after
tissues equilibrate with N2, what occurs when attempting to ascend back to sea level?
ascending will result in the pulmonary, blood, and tissue gases attempting to re-equilibrate quickly with progressively lower pressures toward sea level
If ascent is too fast:
- the tissue gases (especially N2) do not have sufficient time to re-equilibrate with the lower atmospheric pressure
- results in N2 pressure gradient between the tissues and blood
- causes formation of ‘N2 bubbles’ in the blood due to rapid equilibration
N2 re-equilibrates slower because?
N2 is relatively more in tissues (particularly fat) than in plasma → diffusion out of tissues into blood occurs more slowly than N2 movement from plasma
effect of Nitrogen bubbles
Nitrogen bubbles can block blood vessels (air emboli) and/or result in characteristic clinical symptoms of decompression sickness
= joint pain (i.e. the bends) to death (blockage of large blood vessels)
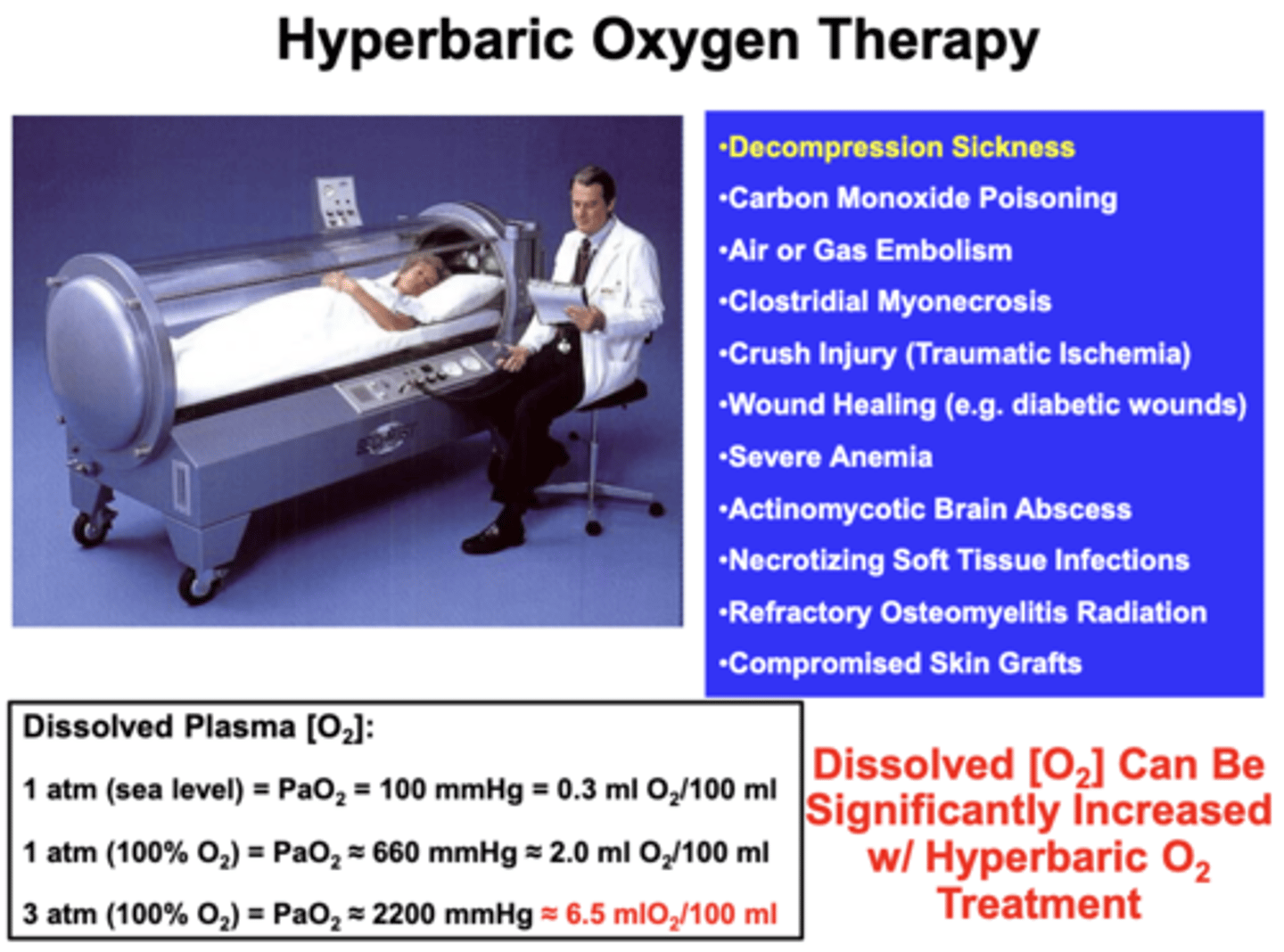
Hyperbaric medicine
AKA Hyperbaric Oxygen Therapy (HBOT)
- medical use of oxygen at a level higher than atmospheric pressure
- PO2 can be raised significantly above maximum values achievable at sea level
- early use was in the treatment of decompression sickness, but has shown great effectiveness in treating conditions such as gas gangrene and carbon monoxide poisoning
equipment required:
- a pressure chamber, which may be of rigid or flexible construction
- a means of delivering 100% oxygen