environemntal pollutants and toxicological chem
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
pollutant
a substance or effect that adversely alters the environment by changing the growth rate of a species, interferes with the food chain, is toxic, or interferes with health, comfort, amenities or property values of people
which sphere is pollution the focus
anthrosphere (pertains to concerns with human activity)
surface/ groundwater contamination
pollutants flow from source to sink
atmosphere → soil → groundwater OR surface water
concerns with water quality
human health and welfare
health of aquatic ecosystems
inorganic pollutants
natural or anthropogenic sources
ex: arsenic in drink water in india and bangladesh is natural due to geological formations containing arsenic
most important difference between organic and inorganic pollutants
inorganic = can never be destroyed because they are chemically reducing elements
organic = can be broken down by various techniques such as UV light
ground water contaminants
fertilizers/pesticides
pharmaceuticals and their metabolites
drugs releases through urine
Dense non aqueous phase liquid (DNAPL)
density > 1g/mL → denser than water → flows down into the water (can cause layer to form across the bottom of the water)
Light Non Aqueous Phase liquid (LNAPL)
density <1g/mL → less sense than water → floats on the surface of water (problem with diffusion and leading to volitization)
solubility
high solubility on water is most dangerous as it can be carried downstream
Kow
inversely proportional to solubility (high Kow = not very soluble)
high Kow is dangerous bc it can build up in tissues by easily hoping across membranes into the cell
octanol water partition coefficient → measures ratio of how a chemical splits between oily and water environments
bio concentration factor
the tendency for a chemical to bioaccumulate
[chemical] in organism / [chemical] in water column
sewage sludge
produced in waste water treatment and is disposed into the ocean, landfills, is incinerated or used in soil → can contain nutrients but also contains environmental pollutants
industrial waste
coal combustion fly ash (CFA) is a by product of burning coal at plants and can build up at a large scale containing high levels of contaminants that have drinking water and ecological impacts
chemical composition of coal fly ash
crystalline phase → high potential for leaching of metals (big problem)
glasseous phase → lower potential for leaching of metals
agriculture
contaminates water with fertilizer runoff causing [O2] to become too low to support aquatic life → increase numbers of dead zones
natural disasters
ex: hurricanes can rupture tanks and cause oil and chemical spills in neighborhoods
chemical disasters (man made since WW1)
accidents with sudden catastrophic release
DIRECT contamination of food and drinking water → health problems over days/weeks
INSIDIOUS contamination of food and water supply → health problems over months/years
worlds worst industrial disaster
india 1984 → 40 tons of CH3NCO was released when water entered the tank → exothermic reaction → increased pressure → tank ruptured and killed many due to complications of exposure
point sources
RELATIVELY EASY TO CONTROL
landfills (emit methane)
underground storage tanks
septic systems
factories, industrial and military installations
snow dumps )contain salt with chemicals that can leach when snow melts)
non point sources
DIFFICULT TO CONTROL/HARDER PROBLEM TO SOLVE
agriculture fertilizer
pesticide applications
road deicing salts
emissions from vehicles
national reports on human exposure to environmental chemicals
~40 organic pollutants and ~10 metals detected and quantified in blood
reports are done to see which chemicals, how much, adverse health effects,, establish reference ranges, make efforts to reduce exposure, look at which groups are exposed
reports have seen a decrease in lead in blood since banning lead in gasoline production
toxicology
the study of harmful interactions between chemicals and biological systems
→ we need this as new chemical and existing chemicals continue to be produced at large amounts increasing exposure to all organisms
toxicologists
collect data on toxicity of compounds, gain knowledge on mechanisms used to produce toxic effects, make responsible predictions about hazards for human populations
types of toxic substances
drugs
food additives/contaminants
industrial chemicals
environmental pollutants
natural toxins
household poisons
drugs
have highly potent activity in biological systems, toxicity is dose dependant
→ must also consider drugs used in veterinary practice as humans can be exposed through meat consumption
example → roxarsone
a feed additive used in chickens to increase growth and egg production
→ not toxic on its own but can be converted to arsenate within 10 days when exposed to air, water, etc → chicken litter spread as agriculture fertilizer → humans exposed to arsenate
food additives/contaminants
food additives = change the colour, flavour, prevent spoilage → usually have low biological activity
contaminants = many potentially toxic substances naturally occurring in food ex: Japanese pufferfish
industrial chemicals
hazards at workplace where they are used, formulated or manufactured → exposes workers and can have long term affects (accumulation in the body over a long period of time)
environmental pollutants
from industrial processes or deliberate release into the air, ocean, rivers, land ex: pesticides and shooting ranges (pb ammunition)
household poisons
pesticides, drugs, solvents, cleaning products → can cause eye and skin irritation
example → paraquat
a herbicide that kills by interfering with photosynthesis
→ can be fatal in human poisonings involving deliberate ingestion, if not fatal causes serious lung and kidney damage
why is paraquat so toxic
accumulates in your lungs as it has a similar structure to diamines which are naturally made in the body therefore the body lets in the toxin due to similar structure
once in the body it builds up and at sufficient concentrations is toxic to alveolar cells (in the lungs)
acute exposure
a high dose enters the system at once (inhalation of a chemical)
chronic exposure
accumulates in your system over a period of time (exposure through drinking contaminated water)
example of Hg exposure in San Fran
wind blowing from the ocean to research site = less [Hg] in air
wind blowing from city or research site = more [Hg] in air
types of poisonings
accidental → usually children and elderly
intentional
4 phases of toxic compound disposition
absorption
distribution
metabolism
excretion
absorption
involves passage across cell membranes in order to exert toxic affect
distribution
via the blood stream by binding to plasma cells
metabolism
leads to more polar metabolite and potential molecular weight increase
excretion
through urine, feces, lungs, breast milk → molecular weight plays an important factor
cell membranes
are selectively permeable only allowing certain substances to pass through based on:
size
lipid solubility
structural similarity
polarity/charge
3 requirements for passive diffusion
the most important mechanism of absorption of toxic compounds
concentration gradient
molecule must be lipid soluble
molecule must be non-ionized
where are weak acids absorbed
stomach
where are weak bases absorbed
intestine
rate of diffusion
FICKS LAW → K x A (C2-C1)
where A is surface area
5 requirements for active transport
specific membrane carrier
metabolic energy for operation
saturable at high [substrate]
transport against concentration gradient
similar substrate may compete for re uptake
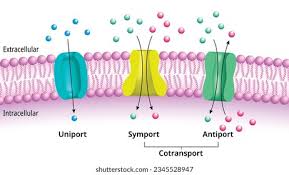
3 types of transporters/carriers
uniport
symport
antiport
→ 2 and 3 are cotransporters
metabolism/biotransformation reactions
get rid of foreign chemicals such as hydrophobic organic compounds, the body attaches hydrophilic groups such as glucuronide sulfate to decrease log Kow and make the compound more soluble
glutathione (GSH) conjugation
found in mM conc in all mammalian cells, reacts either chemically or enzymatically to GS conjugates to be subsequently excreted into bile by ATP pumps to be excreted from the body
types of toxic response as a result of human exposure
7 different ones (direct action, biochemical lesions, pharmacological, immmunotoxicity, tertogenicity, genetic toxicity, carcinogenicity)
direct toxic action
tissue lesions, necrosis and apoptosis
biochemical lesions
interferes with vital functions such as respiration
pharmacological/physiological
affect a particular body function such as blood pressure
immunotoxicity
immune/allergic reactions
tertogenicity
affects embryo development
genetic toxicity
causes genetic mutations
carcinogenicity
produces tumours
selective toxicity
different species of animals and cells have differences in susceptibility and reactions therefore it is hard to predict toxicity in humans using another species
why does selective toxicity exist
differences in absorption, distribution, metabolism, excretion and receptor types
examples → norbormide and penicillin
norbormide - selectively kills rats as they have a receptor in smooth muscle that humans lack
penicillin - interferes with biosynthesis of the bacteria cell wall therefore the bacteria dies if it cant form its cell wall
relationship between dose and response in toxicity
response = death (all or nothing) or pathological lesions (graded responses)
dose = mg of substance / kg body weight
*toxicity dependant on dose and structure
LD50
dose of substance that kills 50% of animals in a given time (not an exact value therefore questions usefulness)
what is LD50 used for
used as a comparison tool to get an idea of how toxic a compound is relative to others → depends on route of administration
botulinum’s toxin (protein)
produced by nature in anaerobic bacillus clostridium botulinum → caused botulism which has adverse affects to processes in the brain → one of the most potent toxins known, found in improperly canned foods
mechanism of toxicity
exposure is rarely limited to a single substance, is often a mixture and the toxicity of two compounds can either be additive, synergistic or antagonistic (detoxify each other)
antagonist example → selenite and arsenite or selenite and mercury chrloride
molecules react and have non toxic effect as they stick together so tightly when they react it cannot affect organisms
ricin
a highly toxic plant product used in intentional poisoning
→ unique mechanism → 1 molecule kills one cell by entering the cell and blocking the ribosome therefore inactivating it and killing the cell