Equilibrium
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
What occurs during dynamic equilibrium?
The concentrations of the reactants and products remains constant
This does NOT mean the concentrations are the same, they just don’t change
In a reversible reaction is carried out in a closed container, a state of dynamic equilibrium can be established
The forward and backward reactions occur at the same rate
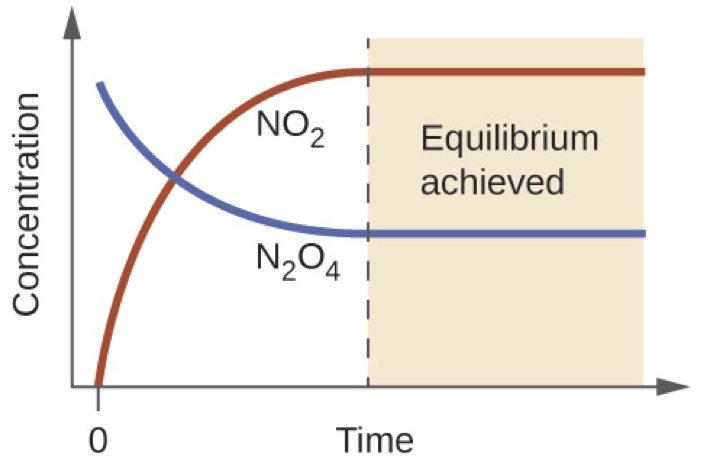
On a a graph showing concentrations of reactants and products, where does the reversible reaction reach equilibrium?
Where the lines remain straight
Remember that when equilibrium is achieved, the concentrations of reactants and products stays constant, but do not have to be the same
What is Le Chatelier’s Principle?
If a factor affecting the position of an equilibrium is altered, the position of equilibrium shifts to oppose the change
How to structure a response to the question of how equilibrium is affected by changes in condition
SHOE
SH= Shift- which direction the equilibrium will shift
O- In order to oppose the change in
E- The effect on the yield/Kc/concentration of products/reactants
How does changing concentration affect equilibrium yield?
Equilibrium shifts right
In order to oppose the increase in concentration to the reactants
The effect is that there is an increase in concentration of the products
Essentially if one side’s concentration increases, equilibrium shifts to the opposite side to oppose the change
How can we tell if a reaction is endo or exothermic?
Negative enthalpy change is exo
Positive is endo
How does increasing/decreasing temperature affect the position of equilibrium?
For an exothermic reaction, increasing temperature shifts equilibrium to the left in order to oppose the increase in temperature
This is because the forward reaction is exothermic, releasing energy, so more energy has to be provided for the endothermic backwards reaction
Decreasing therefore shifts equilibrium to the right
Vice versa for endothermic reactions
How does changing pressure affect the position of equilibrium?
All substances must be in their gaseous state
When pressure is increased, equilibrium favours the side with the least moles of gas in order to oppose the increase in pressure
What are the ideal conditions for and exothermic forward equilibrium reactions where we want to obtain the product? And what problems arise from this? Assume ammonia is being produced.
Low temperature- Rate of reaction is too slow
High pressure- Expensive (and dangerous but AQA and employers don’t care about that lmao)
Expense is due to compressors which use a lot of energy
Because of this we have to compromise
What are the compromise conditions for and exothermic forward equilibrium reactions where we want to obtain the product? Assume ammonia is being produced.
700-750K- compromising between yield and rate of reaction
200 atm- compromise between yield and cost
What do catalysts do and what effect do they have on equilibrium?
They speed up the forward and backward rates of reaction equally
Speeds up the rate at which equilibrium is achieved
Has NO effect of yield
What is the advantage of catalysts?
They can provide the same rate of reaction while letting us compromise for temperature and pressure
They are never used up
What is a Kc expression?
Shows the ratio of the concentration of the products to the reactants for a reaction in homogenous equilibrium
How is a Kc expression written?
Kc= [Products]/[Reactants]
What do square brackets mean in a Kc expression?
Concentration
How do we find the units for a Kc expression?
Write down concentration units (moldm-3) for the number of moles each time for both the reactants and products
Cancel out moldm-3 until all the terms on one or both sides are cancelled out
If terms are on the top then you can leave it as is (and multiply indices if you need)
If the terms are on the bottom you will need to bring them to the top so indices become the opposite sign
If all the terms cancel out, just put ‘no units’
What can we tell about the position of equilibrium and concentrations of reactants and products based on the value of Kc?
If Kc=1 then [reactants] = [products]
The position of equilibrium is central
If the Kc is very large then [reactants] < [products]
The position of equilibrium is to the right
If the Kc is very small then [reactants] > [products]
The position of equilibrium is to the left
What is an ICE table?
Write down the reaction equation
I- Initial concentration
C- Change in concentration
E- Equilibrium concentration
If the reactants increase in concentration, the products have to decrease in concentration and vice versa
We can then use the resulting concentrations to find Kc
If not given the concentration but only the moles, how can we find Kc when the molar ratio of all substances is equal?
Because all molar ratios are the same, we don’t need to worry about volume
If Concentration or […] = moles/volume, if we have the same number of terms on either side, the volumes will cancel out, so we can just use the number of moles
If we are not told the change in moles of any substance within an equilibrium but are given Kc, how can we find the number of moles at equilibrium using an ICE table?
Write an increase or decrease in the number of moles and the no. of moles at equilibrium in terms of x
Put this into a Kc expression and solve for x
You can then use x to add or take away from the initial moles before equilibrium to find the moles at equilibrium
What is the only factor affecting Kc?
Temperature
For all other factors the value of Kc in maintained
How does temperature affect Kc?
For exothermic reactions, increasing temperature shifts equilibrium to the left and the value of Kc decreases
For endothermic reactions, increasing temperature shifts equilibrium to the right and Kc increases
Why doesn’t concentration and pressure affect Kc?
idk im gonna ask miss
What expression is used for homogenous equilibrium involving gasses?
Kp
How do we write out Kp expressions?
We use KPa
We use rounded brackets instead of square brackets as () represents pressure and [ ] represents concentration
What is partial pressure?
The partial pressure of a gas in a mixture is the pressure that gas alone would exert on the sides of it’s container in it’s occupied volume
What is the equation for calculating partial pressure?
Partial pressure = Mole fraction x Total pressure
How can we calculate mole fraction?
Mole fraction= No. of moles of gas x/no. of moles in equilibrium
How do we calculate total pressure?
It is the sum of the partial pressures