isomers
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
2 broad types of isomers
structural and stereo
3 types of structural isomers
chain, position and functional group
2 types of stereoisomers
configurational and conformational
what are configurational isomers
stereoisomers which cannot interconvert
what are conformational isomers
spatial arrangements of molecules through rotation of bonds
types of configurational isomers
geometric and optical
what is a stereogenic centre
a tetrahedral atom with four different substituents is called a stereogenic or chiral centre
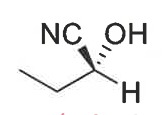
what are enantiomers
show for this molecule + how to redraw to show that they are different
structures that are not identical and non-superimposable mirror images of each other
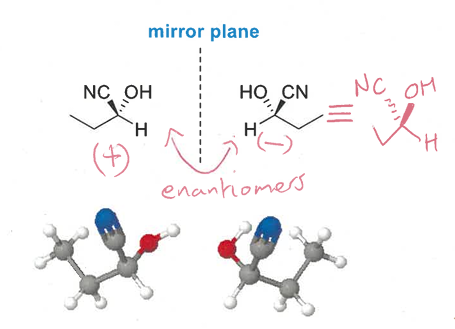
how to distinguish between enantiomers (difference in their behaviour)
both mirror images rotate plane polarised light in equal but opposite directions
other physical and chemical properties are the same
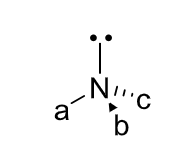
chirality of amines
can be chiral but are not configurationally stable - inversion of the lone pair is fast at room temperature
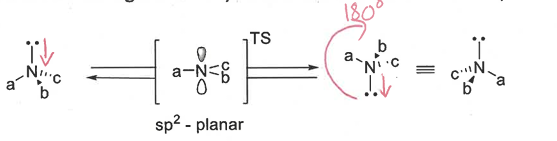
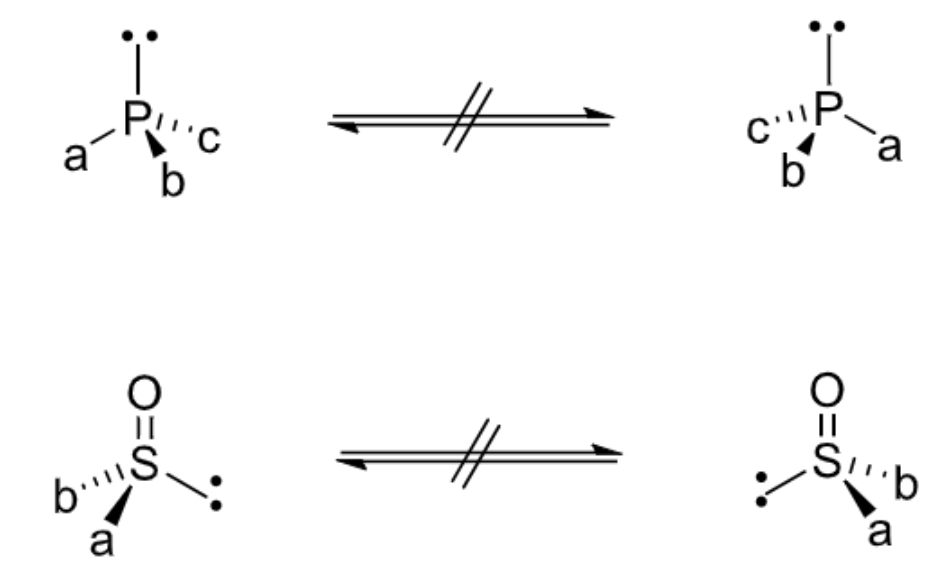
chirality of phosphines and sulfoxides + compare to amines
invert slower than amines so can be isolated as single enantiomers
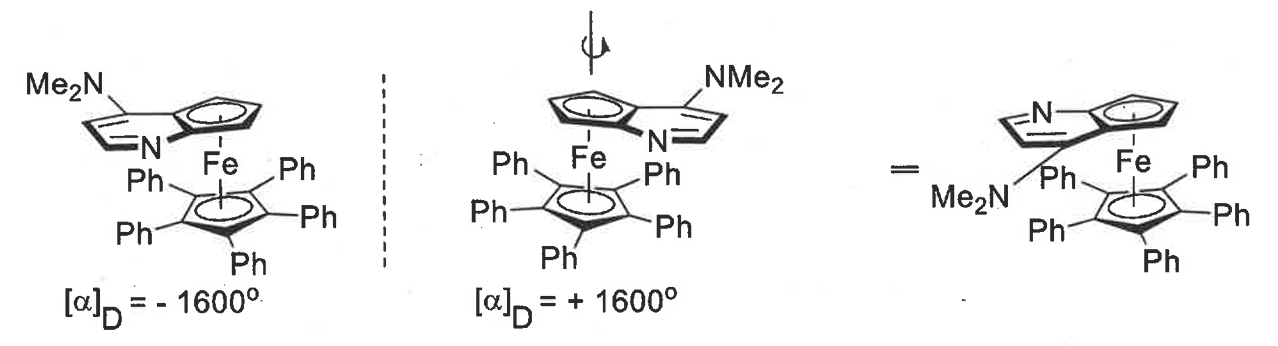
what is planar chirality
features a planar system with a perpendicular substituent
flat ring above and below, Fe bond goes into middle of both rings - rings can spin around on metal axis
the bottom ring is symmetrical but the top ring is not
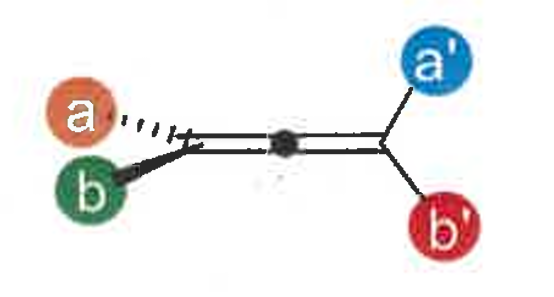
what is axial chirality
a non-planar arrangement of four groups about an axis
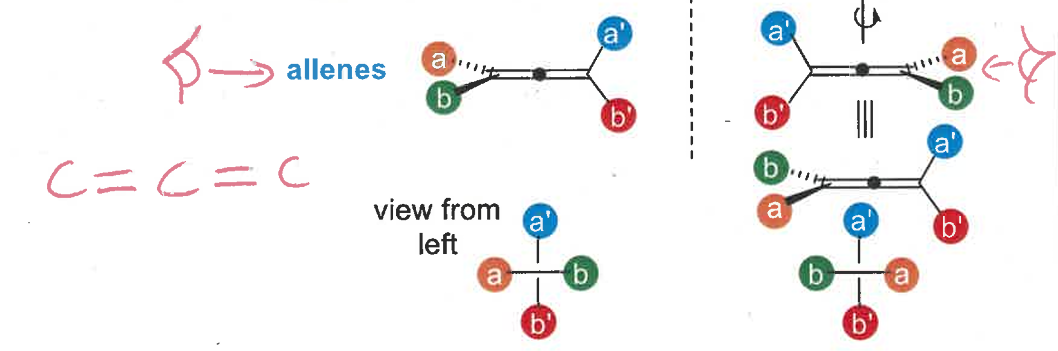
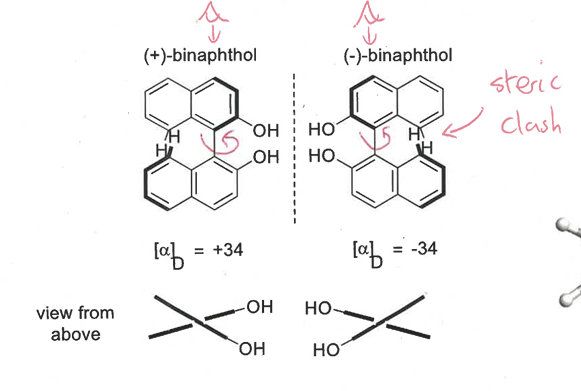
what is atropisomerism
restricted rotation around a chiral axis
as it rotates, the groups hit each other as they are big and bulky resulting in a steric clash
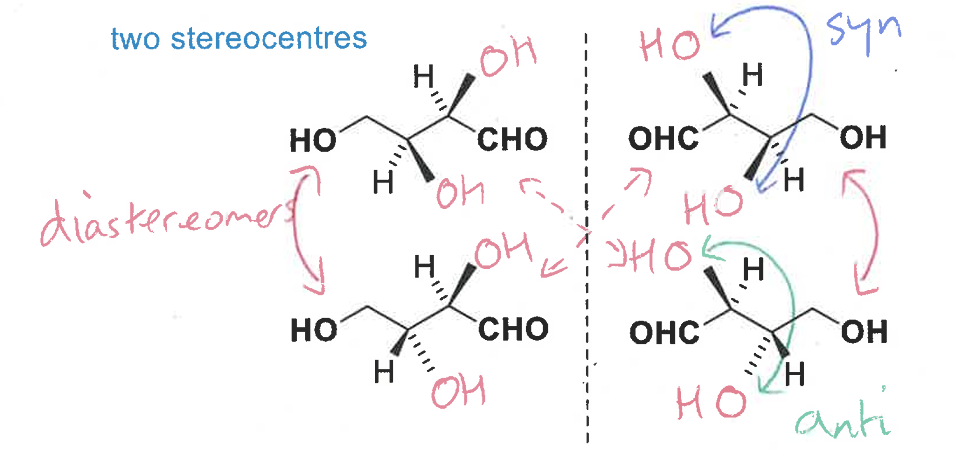
what are diastereomers
what are the types + describe
differences between diastereomers? (think how different to enantiomers…?)
stereoisomers with different configurations at one of more stereocentre
syn - groups on the same face
anti - groups on the opposite face
diastereomers have different physical and chemical properties and can be thought of as different compounds
number of possible stereoisomers and diasteromers for a compound with n stereocentres?

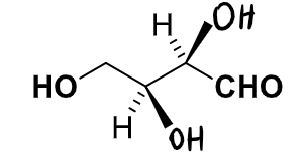
number of stereocentres?
show the diastereomers and label the syn and anti and any other types of isomerism exhibited
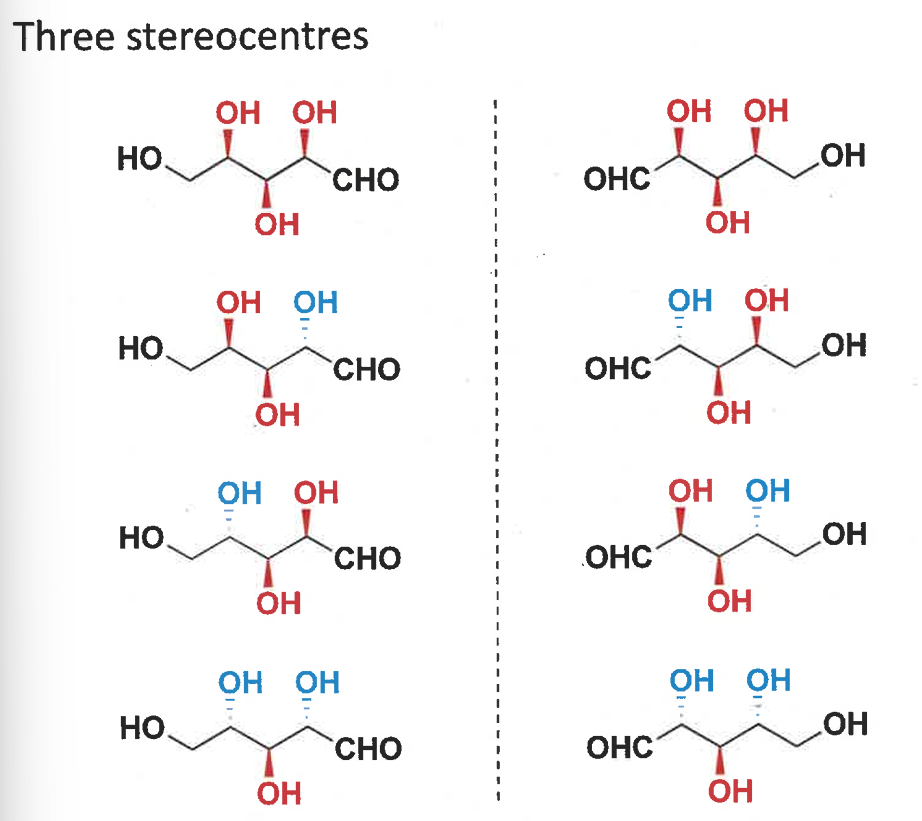
1,2 and 3,4 are pairs of enantiomers
the rest are all diastereomers to each other
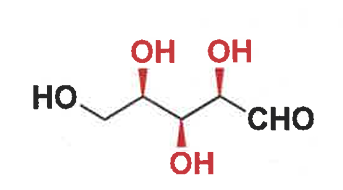
number of stereocentres?
show the stereoisomers
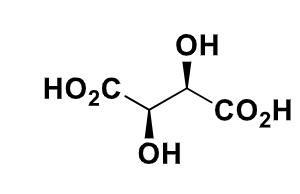
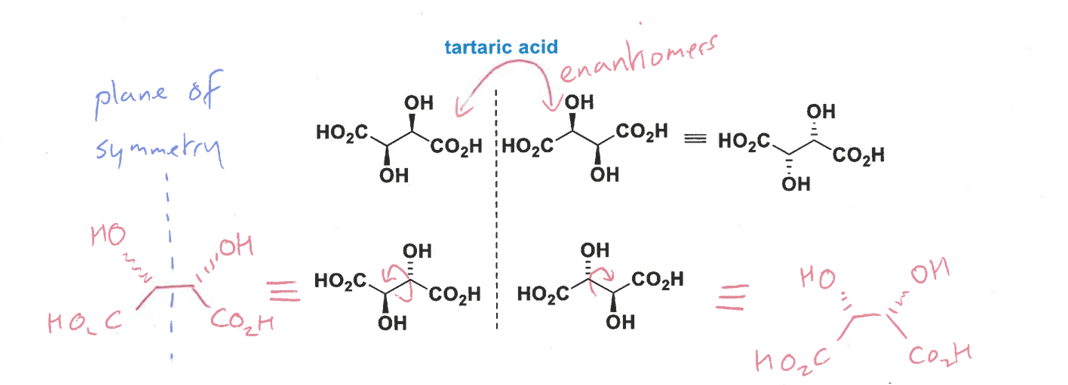
what are meso isomers
explain with the example of this molecule
the stereoisomers have identical substituents but one diastereomer (not really?) is achiral due to a plane of symmetry/point of inversion - so this is a meso isomer instead as an achiral molecule cannot be a diastereomer
what are olefins
alkenes
geometric isomerism in olefins
olefins with non-identical substituents at both carbon atoms can exist as one of two geometrical isomers
nomenclature for 1,2-disubstitued olefins and for more substituted olefins
cis and trans for 1,2-disubstitued
E and Z for more substituted
how are geometric isomers of olefins named (rule)
Cahn-Ingold Prelog rule (for E&Z)
assign which end of the double bond is the highest in priority (this will have 1&2, while the other end will get 1’&2’)
on each carbon of the double bond, assign the priorities of each group
E: two higher priority groups on opposite sides
Z: two higher priority groups on the same side
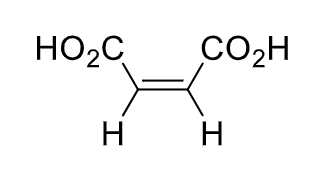
name the isomers
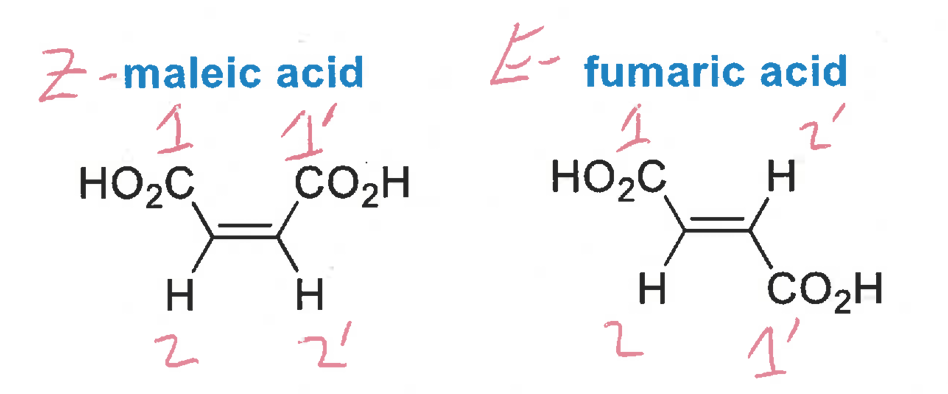
types of geometric isomers for chiral molecules
how can they be determined
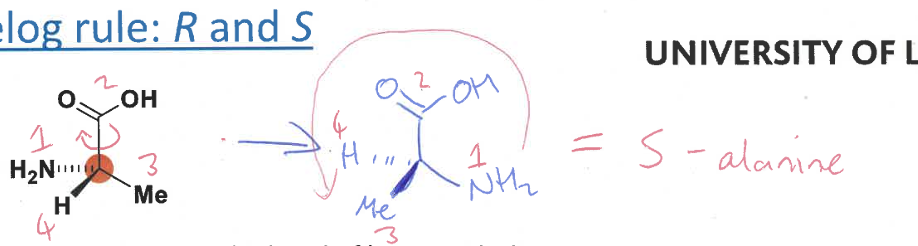
R and S
use Cahn-Ingold Prelog rule again
how to establish priority of substituents for chiral molecules
an element of higher atomic number has priority over an element of lower atomic number (e.g. O > N > C > D > H > lone pair)
a greater number of higher priority atoms takes precedence over a lesser number (e.g. -CHCl2 > CH2Cl)
multiple bonds are regarded as representing two (or three) bonds to the atom of that mass
if steps 1-3 fail to distinguish any substituents, move to the next atom in the ring or chain and repeat. continue this cycle until all substituents are distinguished
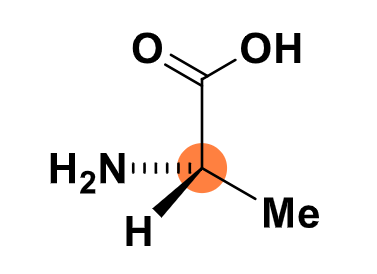
steps in Cahn-Ingold Prelog rule to determine R and S
prioritise the substituents on the molecule from 1 (highest) to 4 (lowest)
redraw the molecule to lok down the bond of lowest priority
determine if substituents 1, 2 and 3 are in a clockwise of anticlockwise order
R: substituents are clockwise
S: substituents are anticlockwise