Thermodynamics 1
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is an isolated system
no exchange of mater or energy with surroundings
whats a closed system
exchange of heat but not matter with surroundings
whats an open system
exchange of matter and energy with surroundings
whats a property
a particular characteristic of the system
what is an intensive property
These are independent of the amount of material in the system
T and p.
what is an extensive property
depend on the amount of material in the system
what is the 1st law of thermodynamics
energy is neither created nor destroyed but just transformed from one kind to another
whats the mathematic equation for the 1st law of thermodynamics
triangleU = q + w
U = energy
Q = heat
W = work
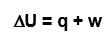
what does the sign of heat (q) tell us
the direction of heat transfer
if heat is transferred out of the substance and q is negative what is the process
exothermic
if heat is transferred into the substance and q is postive then what is the process
endothermic
what happens when heats added to the system
w = 0
-q = w so triangleU= 0
whats the change in enthalpy of a systen
the heat transferred from the surrounding to the system in a constant pressure
whats heat capacity used for
relate change in enthalpy to a change in temprature
whats the standard state of a substance at a specified temprature
its pure form at a pressure of 1 bar
work equation
w = -p2triangleV
w = work
p2 = final pressure
triangleV = difference in volume
what are 3 things that make a process reversible
process produces the maximum amount of work
occurs infinitely slowly
nearly in a state of equilbrium and small changes reverse the direction
what are 3 things that make a process irreversible
less than the maximum quanity of work
occurs rapidly
small changes do not reverse the process
what is an isothermal process
where temprature remains constant
what is a non isothermal process
where temprature changes throughout
are reversible and irreversible processes isothermal or non isothermal
isothermal