2 liver system and diseases
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
What is the first phosphorylation step in the activation of antiviral nucleosides?
Catalyzed by nucleoside kinase
This is the rate-limiting step
What enzyme performs the second phosphorylation of antiviral nucleosides?
Nucleoside monophosphate kinase
Converts nucleoside monophosphate to diphosphate
What enzyme is responsible for the third phosphorylation step of antiviral nucleosides?
Nucleoside diphosphate kinase
Converts diphosphate to triphosphate
What do nucleoside triphosphates compete with in viral replication?
Compete with deoxynucleotide triphosphates
Compete for binding to viral reverse transcriptase
How do nucleoside analogues cause viral DNA chain termination?
They are incorporated into viral DNA
Lack proper 3’-OH group for elongation
Result in premature chain termination
How do antiviral nucleosides affect RNA and protein synthesis?
Reduce synthesis of viral RNA
Decrease viral protein production
Which interferon is used for the initial treatment of chronic hepatitis in adults?
Interferon alpha-2a
Used in chronic hepatitis cases
How does interferon alpha-2a mimic natural immune responses?
Mimics glycoprotein cytokines produced by virus-infected cells
Binds to cell surface receptors
Inhibits viral replication
Promotes viral clearance from hepatocytes
What is the typical administration schedule for interferon alpha-2a?
Subcutaneous (s.c.) injection
3 times per week
Treatment duration: 4–6 months
How is interferon alpha-2a metabolized, and what is its half-life?
Metabolised in the kidneys
Short half-life: 3–4 hours
What is PEG-interferon and why is it used?
PEG = pegylated interferon
Has an extended half-life
Allows for less frequent dosing
What are the common side effects of interferon therapy?
Headache
Myalgia (muscle pain)
Tremors
Fever (typically 4–6 hours post-administration)
What delayed adverse effect is associated with interferon treatment?
Bone marrow suppression
Occurs after prolonged treatment
What is the underlying cause of autoimmune hepatitis?
Autoantibodies target hepatocytes
Who is most commonly affected by autoimmune hepatitis?
Young to middle-aged individuals
Predominantly affects women
What are the common clinical features of autoimmune hepatitis?
Jaundice
Right upper quadrant (RUQ) abdominal pain
May be associated with other autoimmune diseases
What autoantibodies are associated with Type 1 autoimmune hepatitis?
Anti-smooth muscle antibodies (≈80%)
Anti-nuclear antibodies (≈10%)
What autoantibodies are associated with Type 2 autoimmune hepatitis?
Anti-liver/kidney microsomal type 1 antibodies
More common in children
What investigation confirms the diagnosis of autoimmune hepatitis?
Liver biopsy
What are the treatment options for autoimmune hepatitis, including first-line and corticosteroid-resistant cases?
First-line treatment:
Immunosuppressants:
Corticosteroids (e.g. prednisolone)
Azathioprine
Corticosteroid-resistant treatment:
Cyclosporin
Tacrolimus
Mycophenolate mofetil
What is the definitive treatment for end-stage autoimmune hepatitis?
Liver transplant
What is the main cause of liver-related death in the UK?
Alcoholic Liver Disease (ALD)
Due to excessive alcohol intake leading to progressive liver damage
What are the three stages of alcoholic liver disease?
Fatty liver (steatosis)
Alcoholic hepatitis
Cirrhosis
What happens in the fatty liver stage of ALD?
Alcohol metabolism → ↑ hepatic fatty acid synthesis
Fat accumulation in hepatocytes → steatosis
Reversible with abstinence
What characterizes alcoholic hepatitis?
Excess fat → hepatocyte necrosis → inflammation
Presence of Mallory bodies (damaged keratin)
Giant mitochondria in liver cells
Can be life-threatening
What is the final stage of alcoholic liver disease?
Cirrhosis
Irreversible scarring of liver tissue
Leads to liver failure and portal hypertension
What are the clinical features of hepatic steatosis in alcoholic liver disease?
Often asymptomatic
Mild increase in serum bilirubin
Mild increase in alkaline phosphatase (ALP)
What are the key pathological changes in alcoholic hepatitis?
Hepatocyte swelling (fat and water accumulation)
Cellular necrosis
Neutrophilic inflammatory reaction
Fibrosis begins to develop
What are the clinical manifestations of alcoholic hepatitis?
Range from minimal to severe
Nonspecific symptoms (e.g. malaise, fever, abdominal pain)
Increased serum bilirubin
Increased alkaline phosphatase (ALP)
What causes hepatocellular steatosis in alcoholic liver disease?
Alcohol metabolism produces excess NADH via:
Alcohol dehydrogenase
Acetaldehyde dehydrogenase
Excess NADH leads to:
↑ Lipid biosynthesis
↓ Lipoprotein assembly/secretion
↑ Peripheral fat catabolism
What is the result of alcohol-induced changes in lipid metabolism in the liver?
Lipid droplet accumulation in hepatocytes
Leads to hepatic steatosis (fatty liver)
What are the two histological types of hepatic steatosis?
Microvesicular steatosis
Macrovesicular steatosis
What is the typical histological progression of hepatic steatosis?
Starts centrilobular (around central veins)
Progresses to panlobular (involving entire lobule)
What is the gross appearance of a liver with steatosis?
Large, soft, yellow, and greasy liver
Is hepatic steatosis reversible?
Yes, completely reversible with alcohol abstention
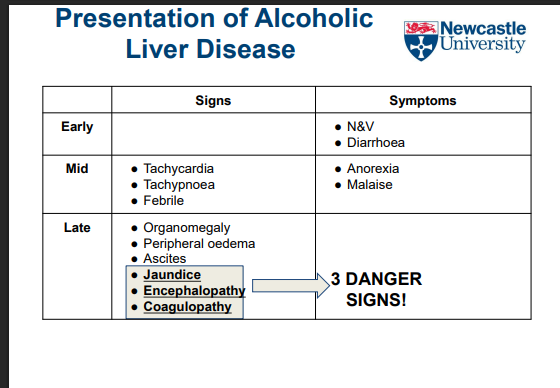
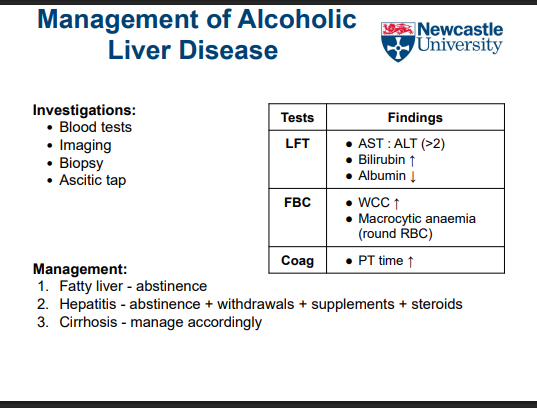
What happens to liver tissue in cirrhosis?
Functional hepatocytes are replaced by non-functional connective tissue
Leads to impaired liver function
How does cirrhosis affect drug elimination?
Reduced drug elimination in liver dysfunction
Higher systemic drug levels for longer durations
May increase drug efficacy or toxicity
how are low clearance drugs affected in cirrhosis?
Little effect until end-stage cirrhosis or liver failure
Clearance remains fairly stable in early disease
What is the impact of portal hypertension in cirrhosis on drug metabolism?
Causes shunting of blood around the liver
Leads to greater oral drug delivery to systemic circulation
Increases systemic levels of high clearance drugs
Does cirrhosis increase susceptibility to idiosyncratic or autoimmune drug reactions?
No,
Increased likelihood of Autoimmune-mediated drug reactions
What are the common liver-related and external causes of cirrhosis?
Alcohol (most common cause)
Drugs and xenobiotics
Chronic viral hepatitis (e.g. Hep B, Hep C)
Autoimmune hepatitis (chronic self-directed inflammation)
What biliary disorders can lead to cirrhosis?
Chronic bile duct blockage
Biliary atresia (congenital)
Primary biliary cirrhosis (cause largely unknown)
Primary sclerosing cholangitis (bile duct narrowing/blockage)
What inherited metabolic diseases can cause cirrhosis?
Wilson’s disease ( abnormal copper accumulation)
Haemochromatosis (abnormal2iron overload)
What are the early liver changes in alcohol-mediated hepatic cirrhosis?
Liver is yellow-tan, fatty, and enlarged
How does the liver appear in advanced alcohol-mediated cirrhosis?
Liver becomes brown, shrunken, and non-fatty
What histological changes occur in alcohol-mediated hepatic cirrhosis?
Fibrous septa thicken and extend through sinusoids
Formation of regenerative nodules trapping hepatocytes
How long does it usually take for cirrhosis symptoms to develop, and what is the early clinical presentation?
Develops over many years
Often largely asymptomatic until advanced stages ……
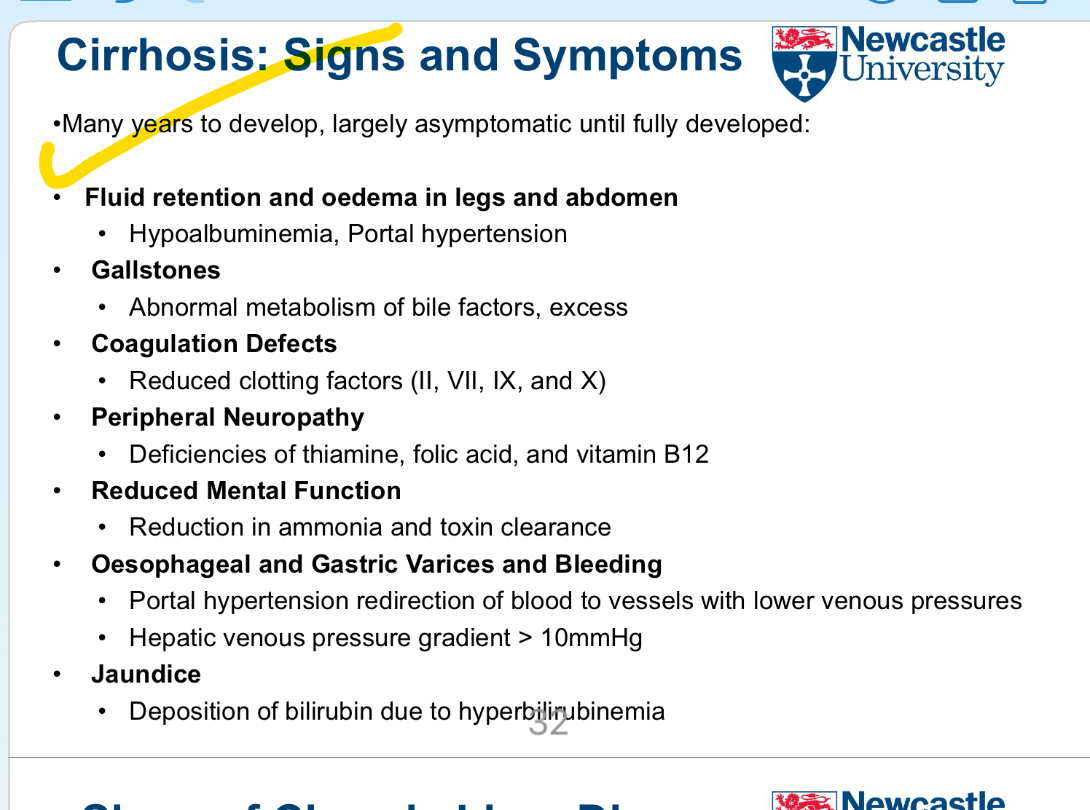
Is there a specific drug therapy for cirrhosis?
No specific drug therapy for cirrhosis itself
Treatment focuses on managing symptoms and complications
What is the most important lifestyle intervention in cirrhosis treatment?
Cessation of alcohol consumption
How is oedema managed in cirrhosis?
Salt restriction
Use of diuretics
How is chronic hepatic encephalopathy treated pharmacologically?
Laxatives (e.g. lactulose) to reduce colonic neurotoxin (ammonia) production
Oral antibacterials (e.g. metronidazole) to reduce bacterial ammonia production
What treatments are used for variceal haemorrhage in cirrhosis?
Correct coagulation defects with platelet transfusion and plasma
Endoscopic variceal injection with sclerosant (induces inflammation)
Endoscopic tissue glue (cyanoacrylate adhesive) to block bleeding vessels
Vasopressin analogues (e.g. terlipressin) cause splanchnic vasoconstriction to reduce portal pressure
What is the leading cause of acute liver failure?
Drug-Induced Liver Injury (DILI)
Does drug-induced liver injury occur in all patients taking the drug?
No, only a small fraction of individuals are affected
How quickly can drug-induced liver injury develop?
Can be gradual, occurring weeks, months, or years after starting therapy
Why is drug-induced liver injury a major clinical concern?
Often life-threatening
Main reason for drugs being removed from clinical development or use
What mechanisms contribute to drug-induced liver injury?
Direct hepatocyte damage
Mitochondrial toxicity
Toxic metabolite activity
Cholestasis
Drug-drug interactions (some predictable)
Idiosyncratic, metabolic, or genetic factors (rare and unpredictable)
What are the three main hepatocyte zones in the liver lobule?
Periportal region (Zone 1)
Mid-zone (Zone 2)
Centrilobular region (Zone 3)
What are the characteristics of the periportal region (Zone 1)?
Highest oxygenation (mainly from hepatic artery)
Least sensitive to ischemic injury
Hepatocytes specialized in oxidative functions:
Gluconeogenesis
Cholesterol synthesis
β-oxidation of fatty acids
Most susceptible to viral hepatitis
What are the characteristics of the centrilobular region (Zone 3)?
Lowest oxygenation (most vulnerable to ischemia)
Hepatocytes specialize in:
Glycolysis
Lipogenesis
P450 drug detoxification
Most affected during ischemic injury