Protease Kinase
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
What are kinases + proteases and what are they important in?
kinases: enzymes that add phosphate groups
proteases: enzymes that cut proteins
important intracellular signalling, which controls how cells respond to signals
What are the steps of TGF-beta signalling pathway?
TGF-beta ligand (red) binds to type II receptor
type II receptor activates type I receptor using phosphorylation (adding a phosphate group)
type I receptor then phosphorylates R-Smad (signal-carrying protein)
phosphorylated R-Smad joins with Smad4
this complex moves into nucleus + binds to DNA-binding partners (ex. Fast-1) to turn on specific genes
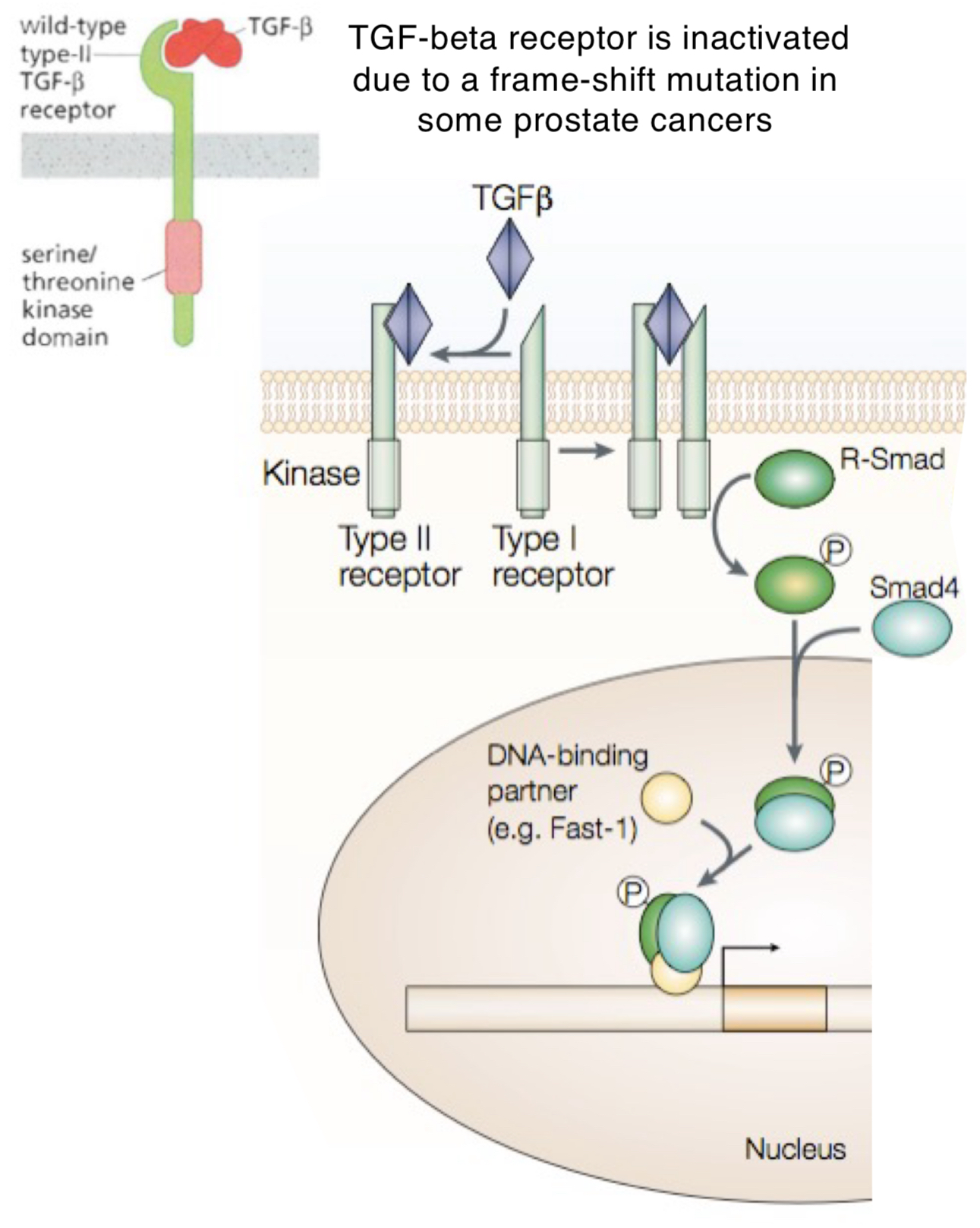
What does a frame-shift mutation do to a TGF-beta signalling pathway?
Inactivates TGF-beta signalling pathway, leading to cancer since TGF-beta usually helps regulate cell growth + prevent uncontrolled division.
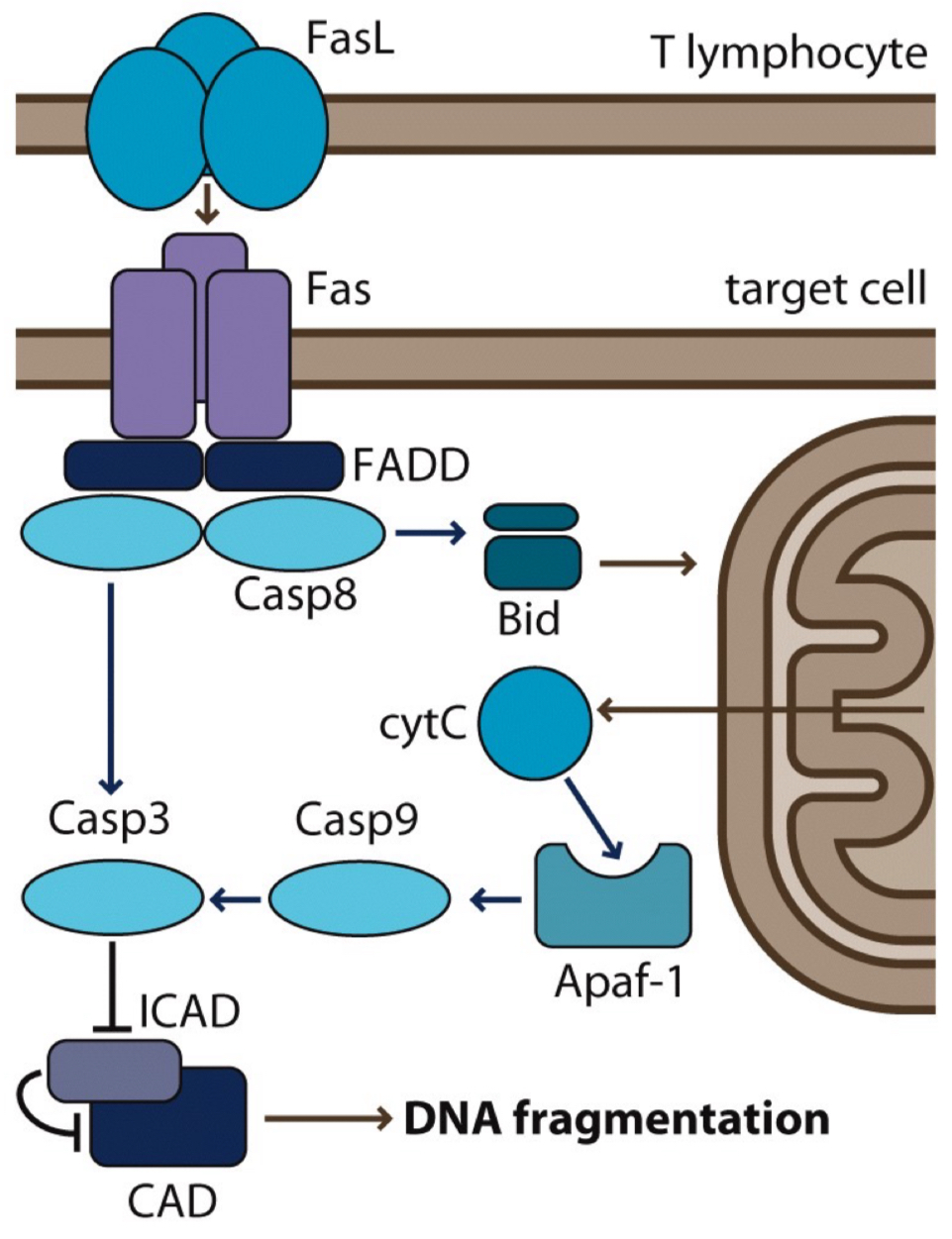
What are the steps of FasL signalling pathway (important for programmed cell death: apoptosis)?
T lymphocyte (immune cell) produces FasL
FasL binds to Fas receptor on a target cell
this recruits FADD, which activates Casp8
Casp8 triggers 2 pathways:
direct activation of Casp3 ➡ DNA fragmentation
activation of Bid ➡ releases cytC from mitochondria
cytC binds to Apaf-1, which activates Casp9
Casp9 further activates Casp3
Casp3 breaks down ICAD ➡ frees CAD
CAD cuts up DNA ➡ cell death
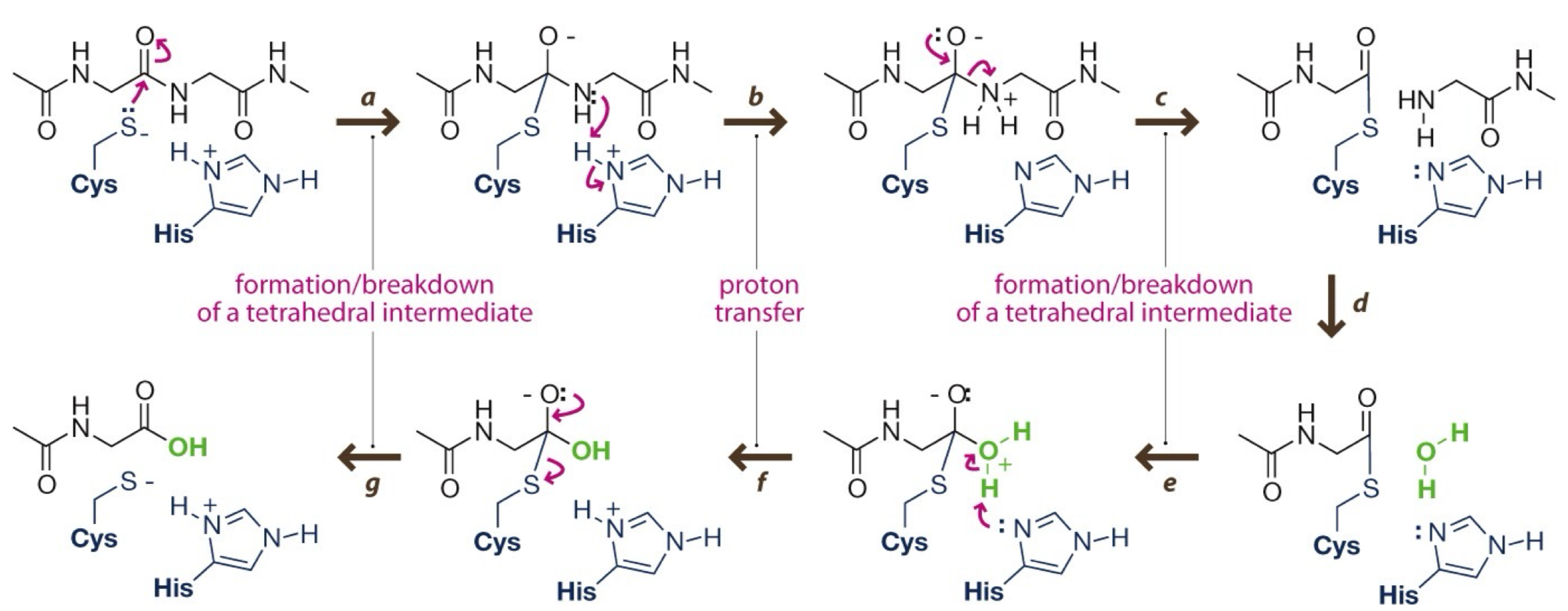
What are the steps of proteases?
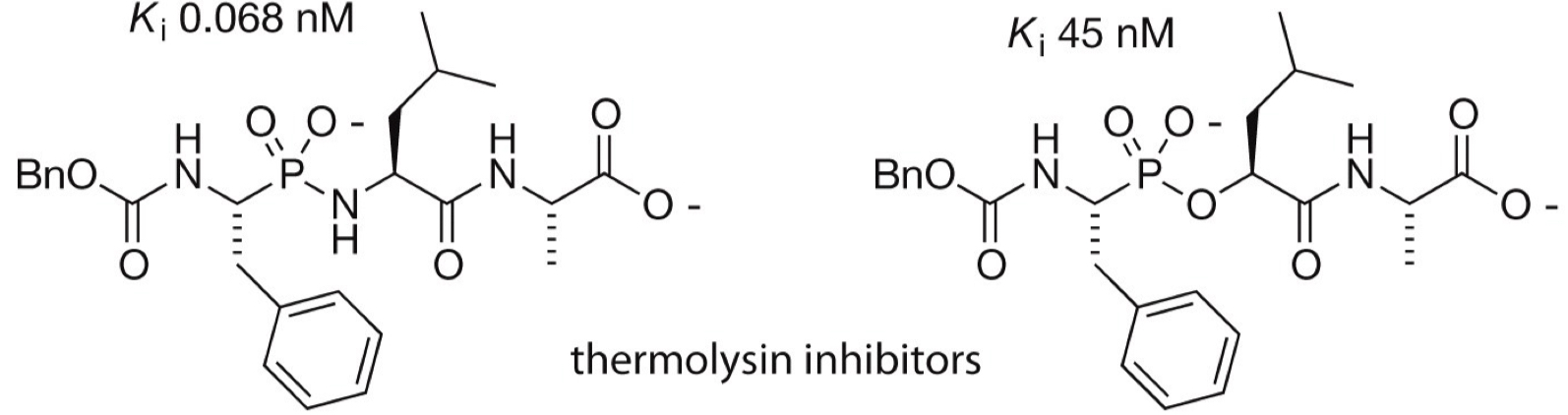
What are thermolysin inhibitors + their shapes?
Mimics tetrahedral state of protease with phosophate/phosphoramidate
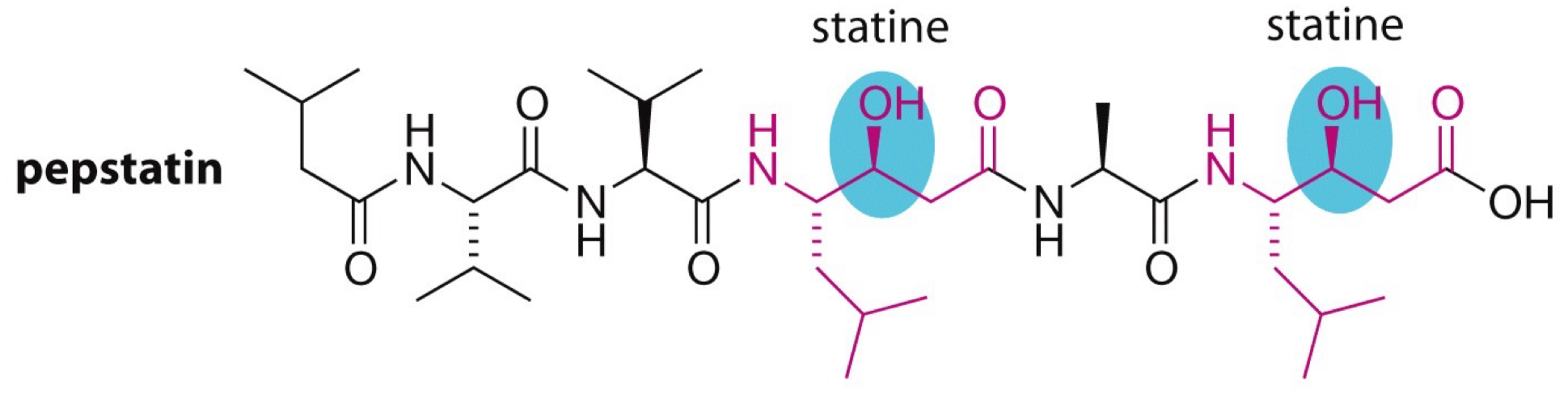
What is pepstatin, its structure, the 2 parts where it’s statine amino acids + 2 parts where carbinols are?
Naturally occurring inhibitor that mimics tetrahedral state of protease with carbinols.
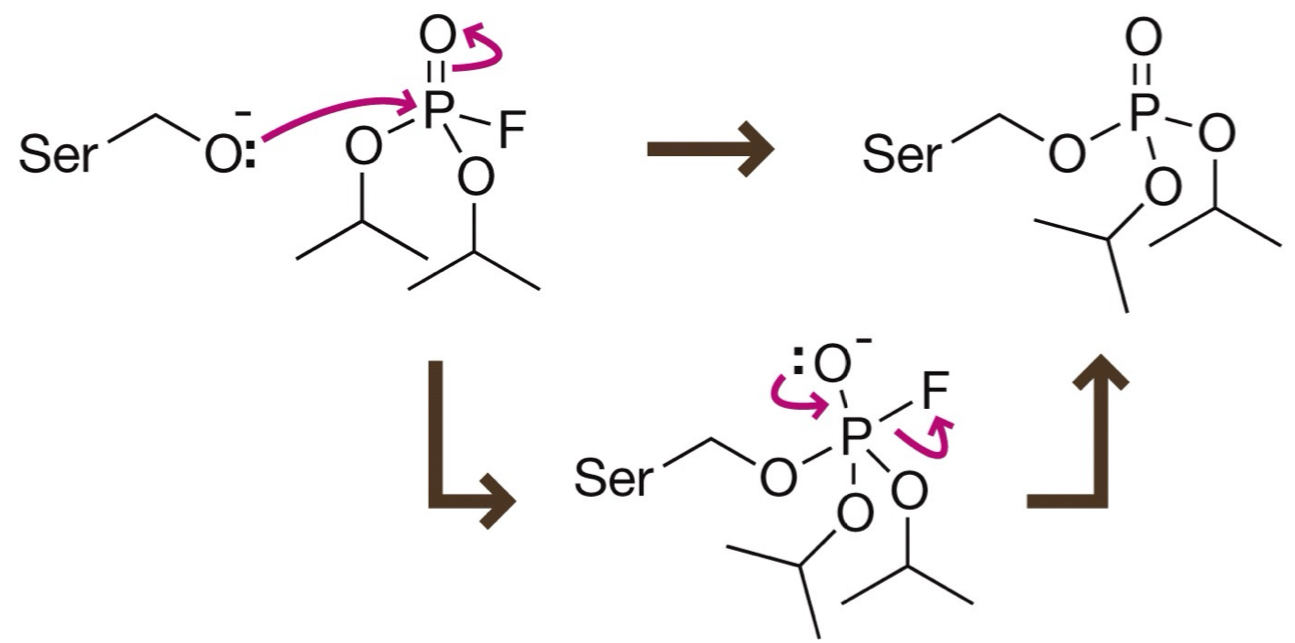
What’s the mechanism for irreversible inhibition where fluorophosphates inhibits serine protease?
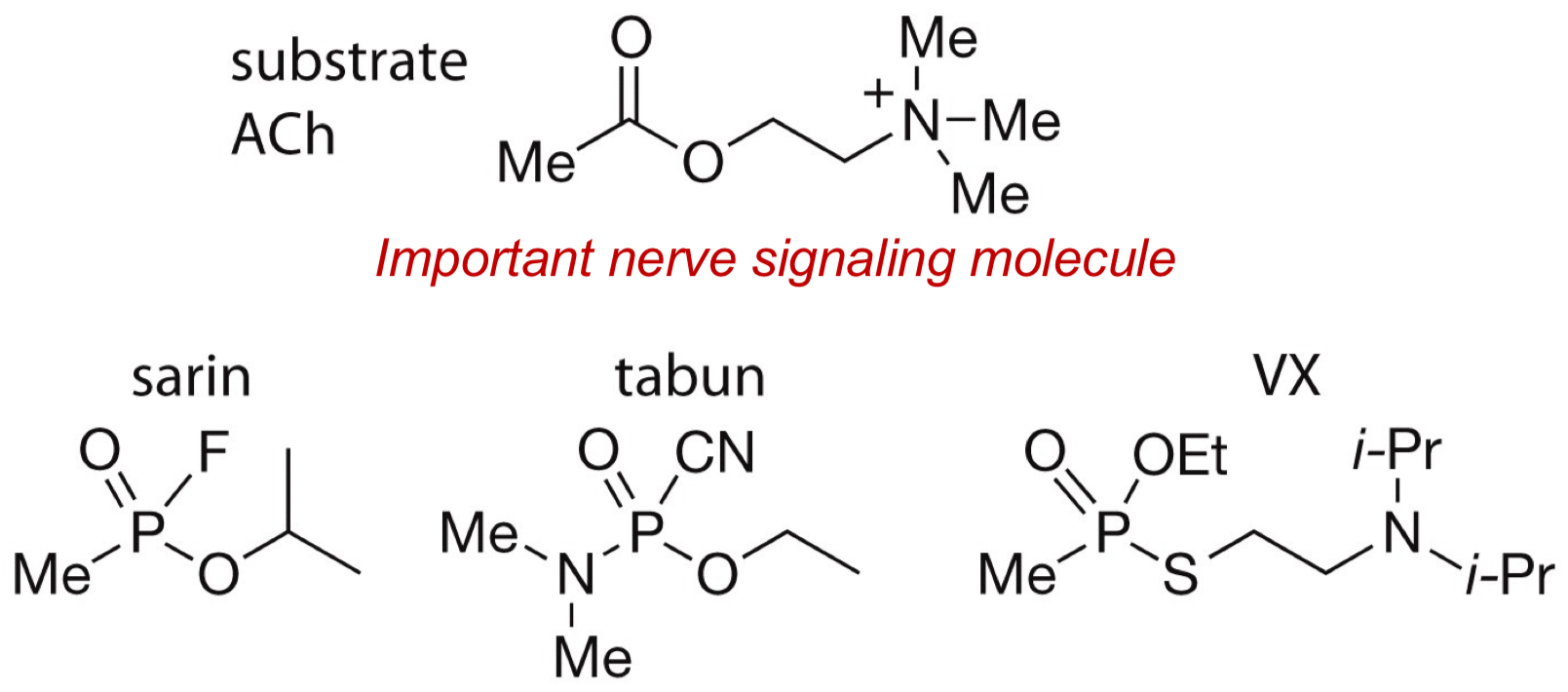
What are the 3 nerve agents that inhibits acetylcholine esterase (important nerve signalling molecule)?
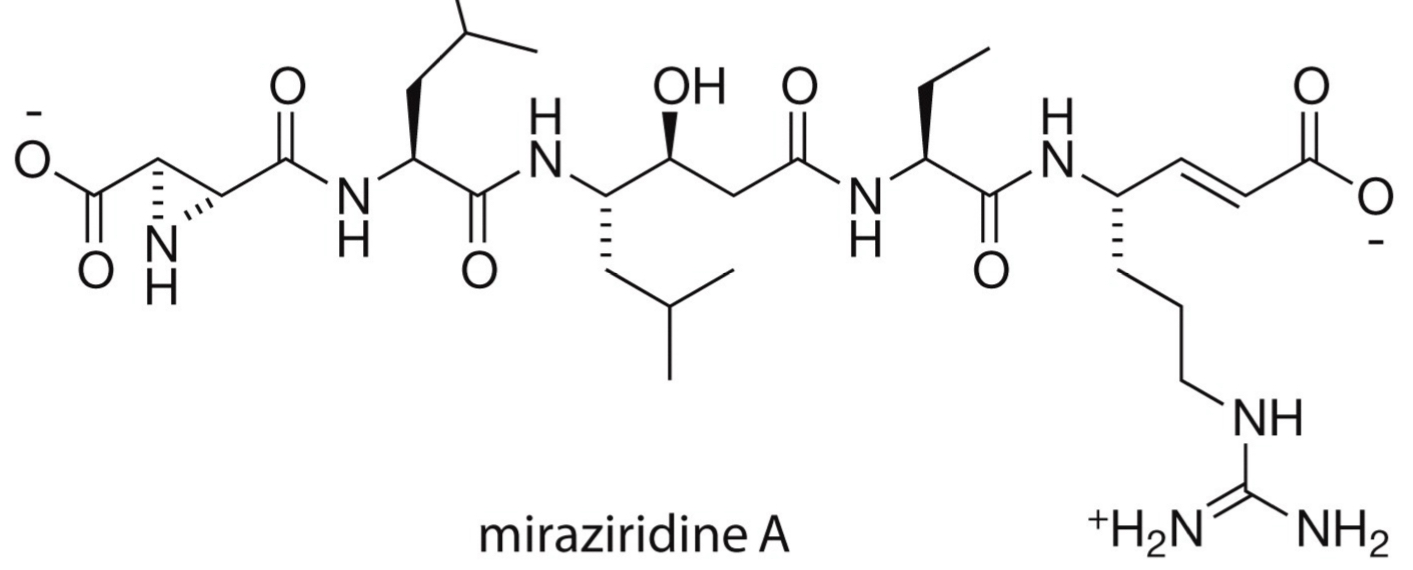
What’s miraziridine A’s structure + how is it a multifunctional (combination) inhibitors?
Simultaneously inhibits 2 or more biological targets.
What are the electrophiles for multifunctional (combination) inhibitors?
alpha, beta unsaturated acid (Michael addition) Aziridine
What type of protease inhibited by such electrophiles?
Mainly cysteine.
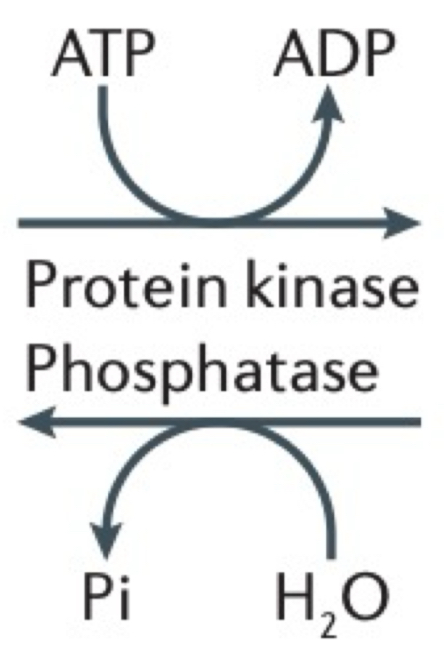
What’s the difference between protein kinase + phosphatase?
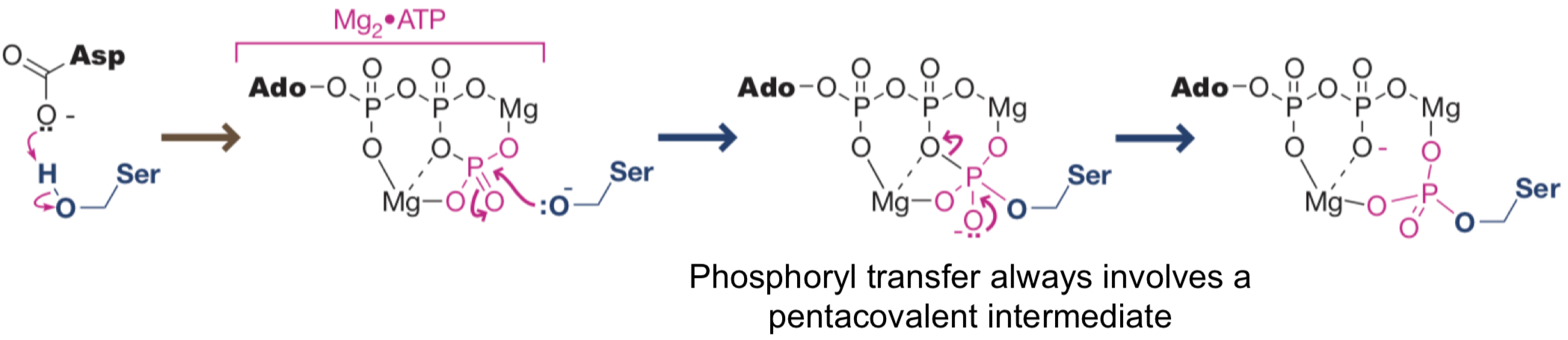
What’s the mechanism for phosphoryl transfer?
What are the 3 main parts of an ATP molecule?
adenine (nitrogenous base)
ribose (sugar)
triphosphate (3 phosphate groups that store energy)
Why do kinases need ATP?
Uses ATP as phosphate donor to phosphorylate proteins, which activates or deactivates signalling pathways.
What is the kinase active site?
Region where ATP binds for phosphorylation, including glycine-rich loop (stabilizes ATP binding) + DFG loop (involved in activation).
What is balanol + its structural features?
natural fungal metabolite that acts as ATP inhibitor by blocking kinase active site
structurally resembles ATP but doesn’t allow phosphorylation ➡ stops signalling pathways
structural features: benzophenone, hydroxybenzamide + perhydroazepane groups
Why do kinases need to be switchable?
switchable: be able to turn on + off in response to cellular signals
crucial for precise control, preventing overactivation, dynamic signaling
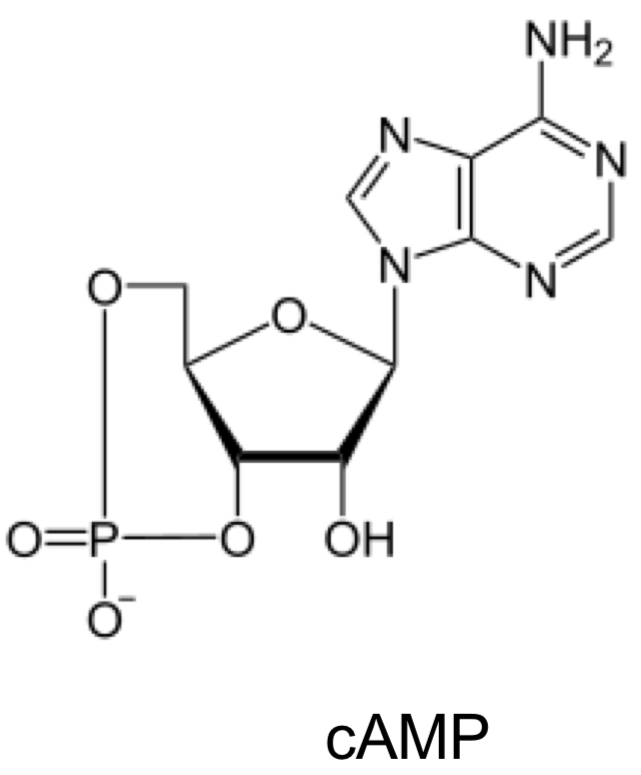
What is cAMP an example of?
How small molecules can activate a kinase by acting as allosteric regulators (bind to site on kinase that changes its shape + function).
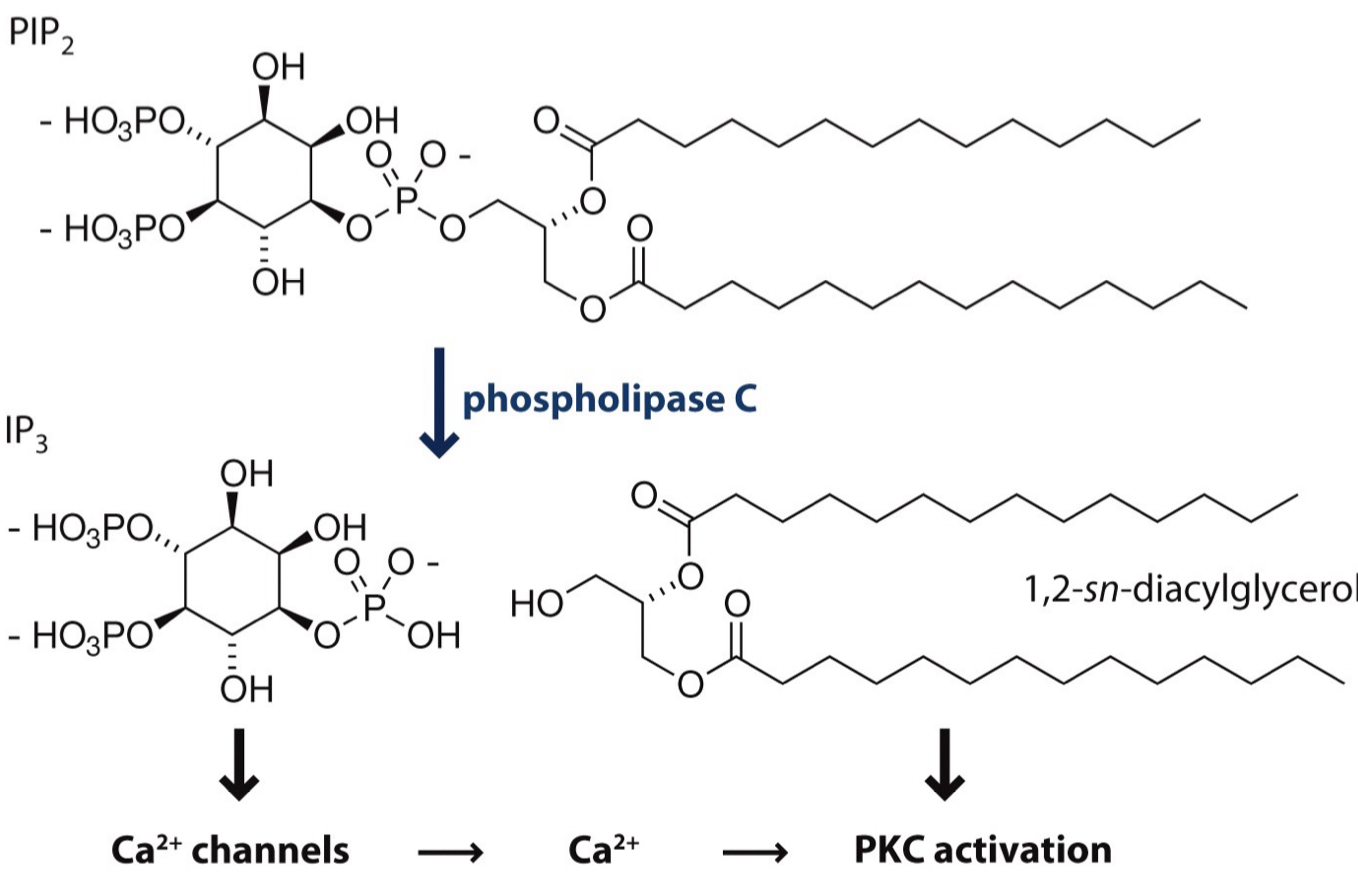
What activates protein kinase C (PKC)?
calcium ions
diacylglycerol (DAG)
How does PKC activation work?
PIP2 is broken down into IP3 + DAG
IP3 releases Ca2+ from storage
DAG stays in membrane + helps activate PKC
PKC moves to membrane, binds DAG & Ca2+ + phosphorylates proteins
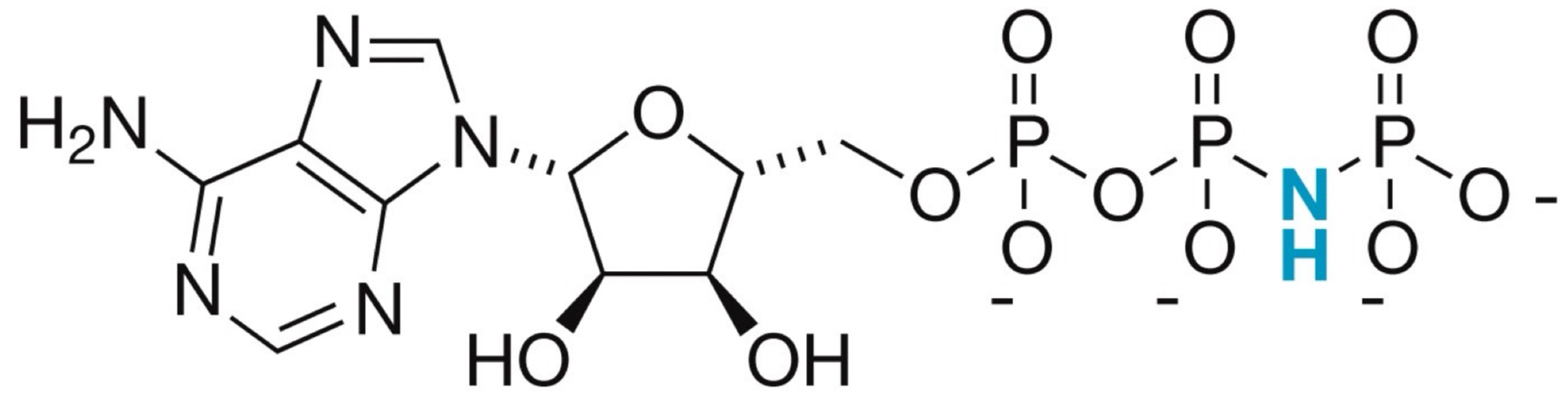
What is the structure of the ATP analog that’s used to crystallize kinase?
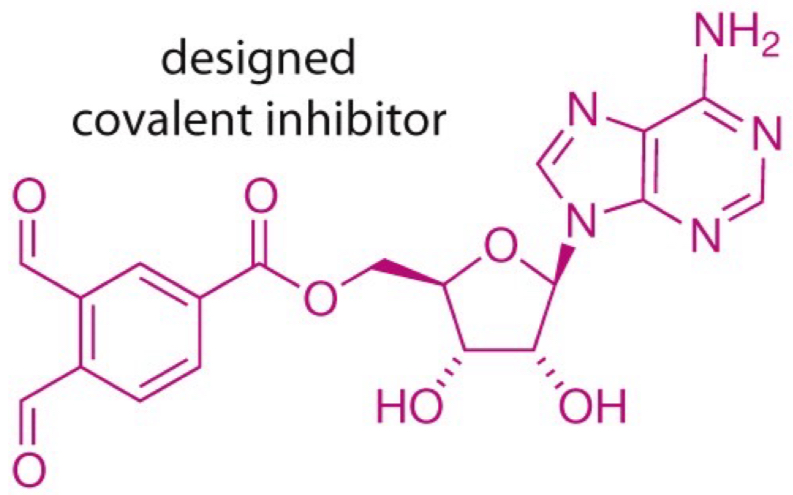
What’s the structure of the designed covalent inhibitor?
What are 3 ways scientists study kinases?
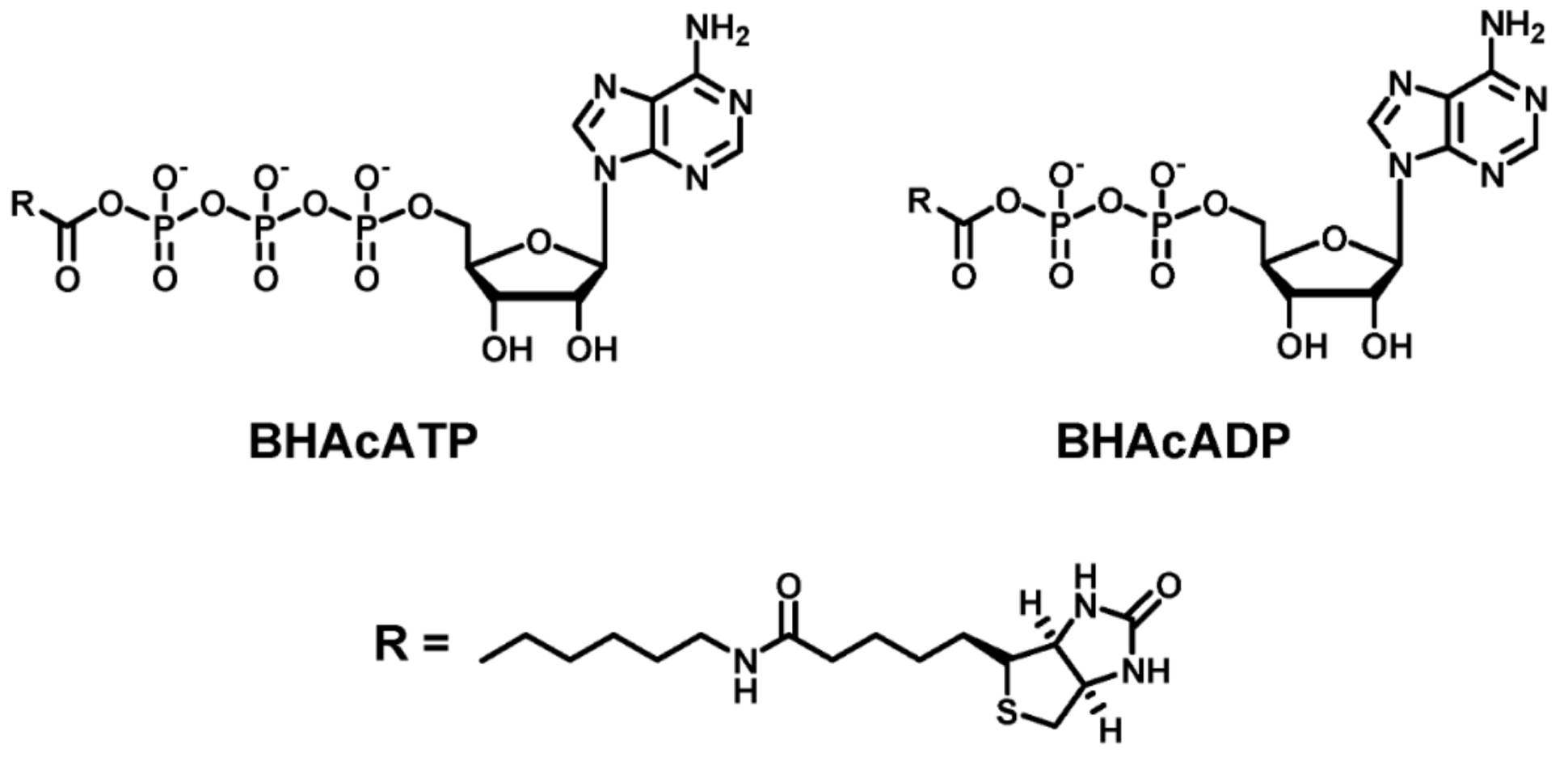
ATP analog binding: uses special ATP that has a biotin tag to see how kinases work
fluorescence tracking: designed peptide or protein substrate that contains special fluorescent tag
when kinase acts on substrate, fluorescence helps track kinase activity
covalent inhibitors: permanently binds to kinase’s active site to prevent it from working
What’s the structure of BHAcATP, BHAcADP + its R group?
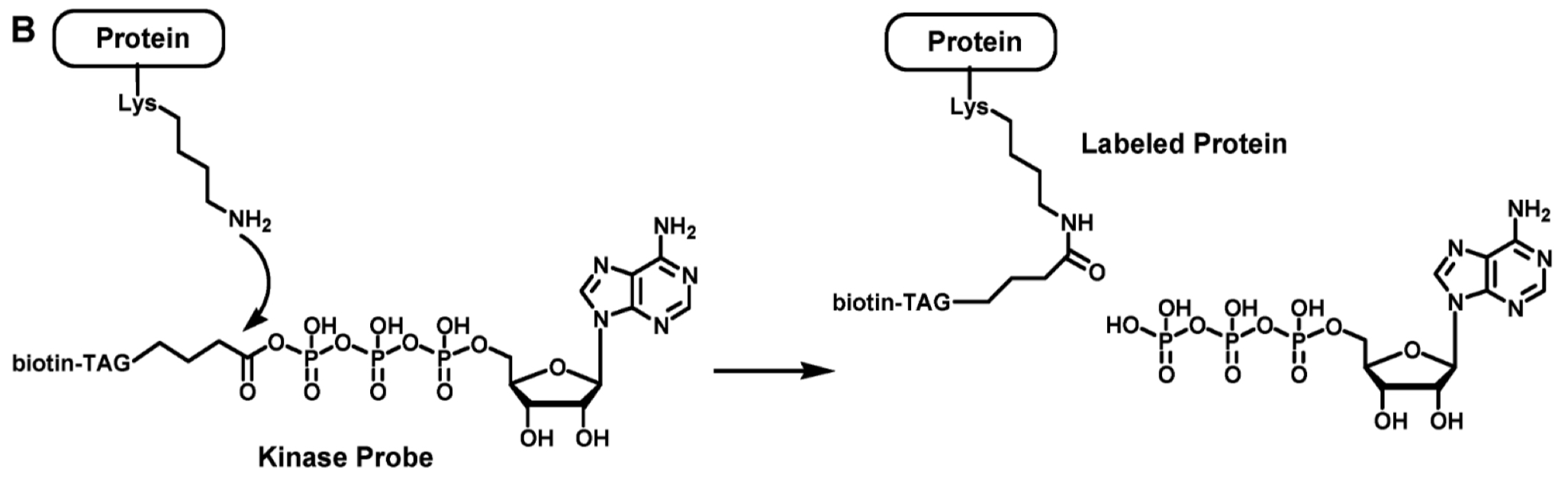
What’s the mechanism of a protein binding to a kinase probe?
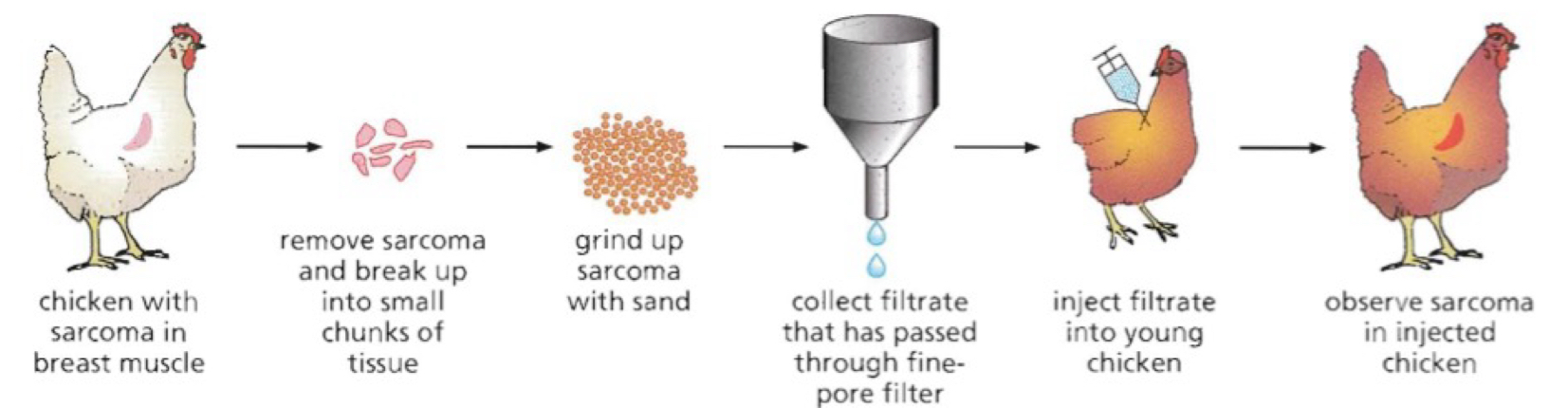
What did Peyton Rous demonstrate about cancer (sarcoma)?
How did Rous sarcoma virus (RSV) evolve from Avian Leukosis virus (ALV)?
RSV evolved when ALV accidentally incorporated the adjacent c-src gene from a chicken, modifying it into v-src.
Why does a virus carry a chicken gene?
RSV evolved from ALV through accidental incorporation of the adjacent c-src gene in an ancient chicken.
What’s the difference between c-src + v-src?
c-src: normal chicken gene
v-src: mutated version carried by rsv that promotes uncontrolled cell growth
key difference: deletion of C-terminal tail
means that v-src is “always on” since it lost ability to be regulated by phosphorylation on C-terminal tyrosine
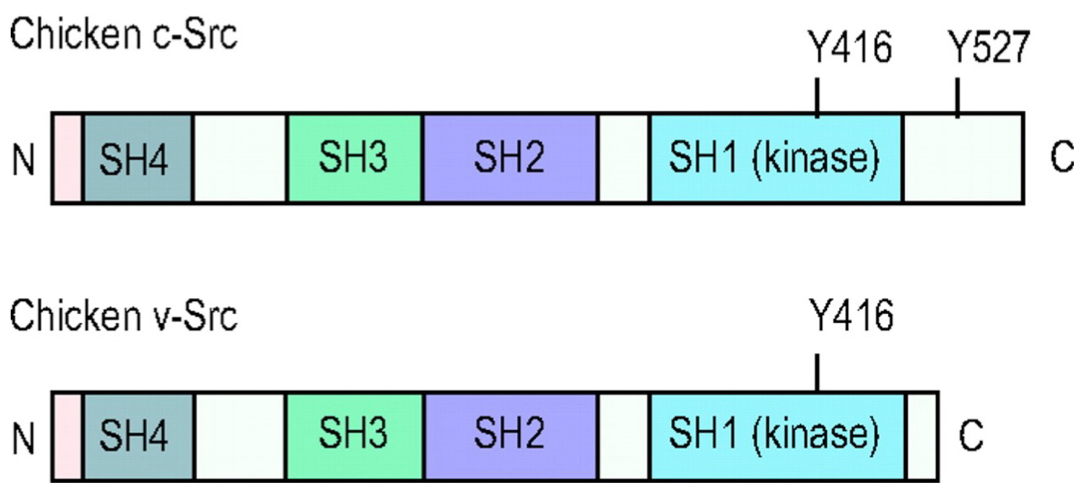
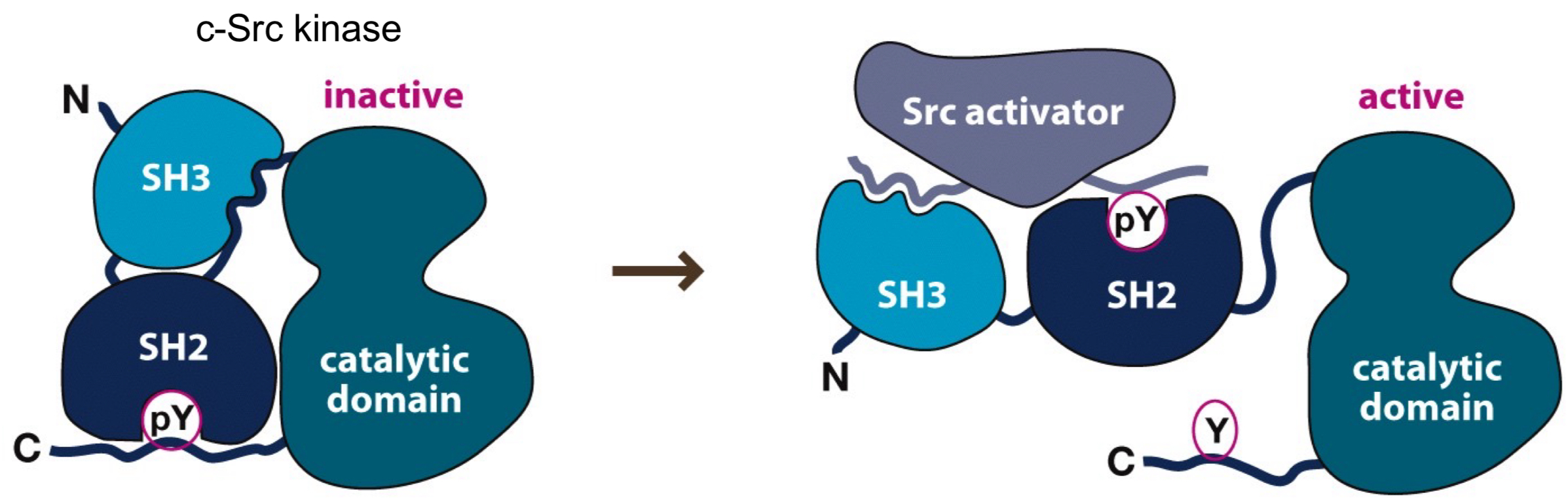
What does it mean when Src is THE archetypical kinase?
Domains of all kinases are named after Src.
Why is Src considered an oncogene?
It regulates multiple cellular processes, and when overactive, it can lead to uncontrolled cell growth.
Why can’t scientists pinpoint a single reason why Src causes cancer?
Src affects many different pathways in the cell, making it difficult to identify one specific mechanism responsible for its oncogenic effects.
What 3 receptors can activate Src?
receptor tyrosine kinases
G-protein-coupled receptors
integrins
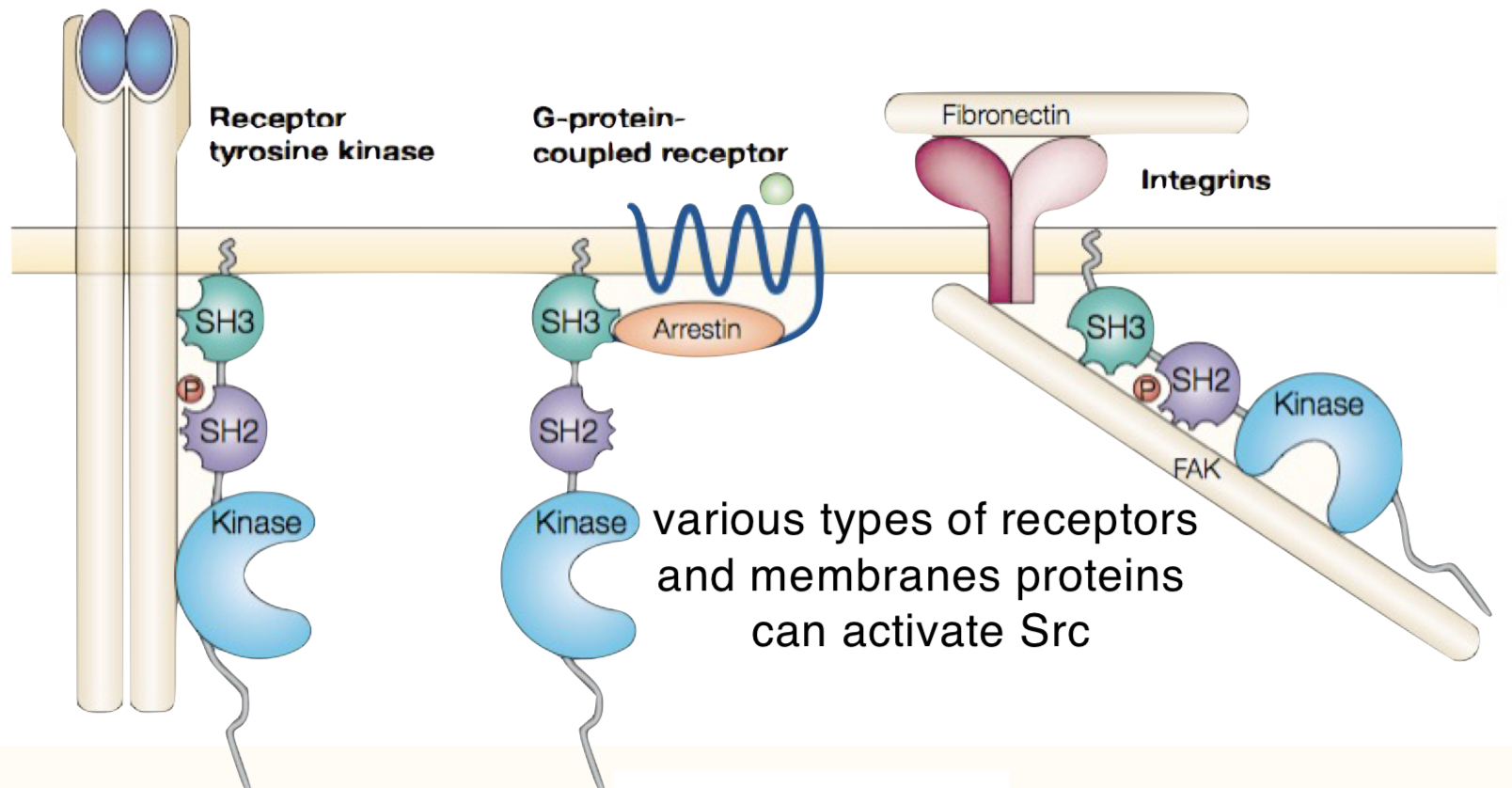
What cellular processes does Src regulate?
motility (cell movement)
proliferation + survival growth (growth + avoiding cell death)
cell-cell adhesion
What are some major pathways activated by Src?
OU3J → Akt → mTOR (controls survival, metabolism, + growth)
Ras → Raf → MEK → MAPK (controls cell division)
Stat3 (controls gene expression)
Ets/TCF, Fos, Myc, Cyclin D1 (regulate cell growth)
There are many kinases in the cell, all catalyzing the same reaction, so how can we determine the substrates of just one of them?
“bump and hole” approach
bump (enzyme mutation): enlarging an amino acid in active site to block normal binding
hole (substrate modification): modifying the substrate to fit only the mutated enzyme
What modifications was made to v-Src to allow it use a special ATP analog?
Mutations at I338 (gatekeeper residue) and V323 allow v-Src to bind N⁶-cyclopentyl ATP instead of regular ATP.
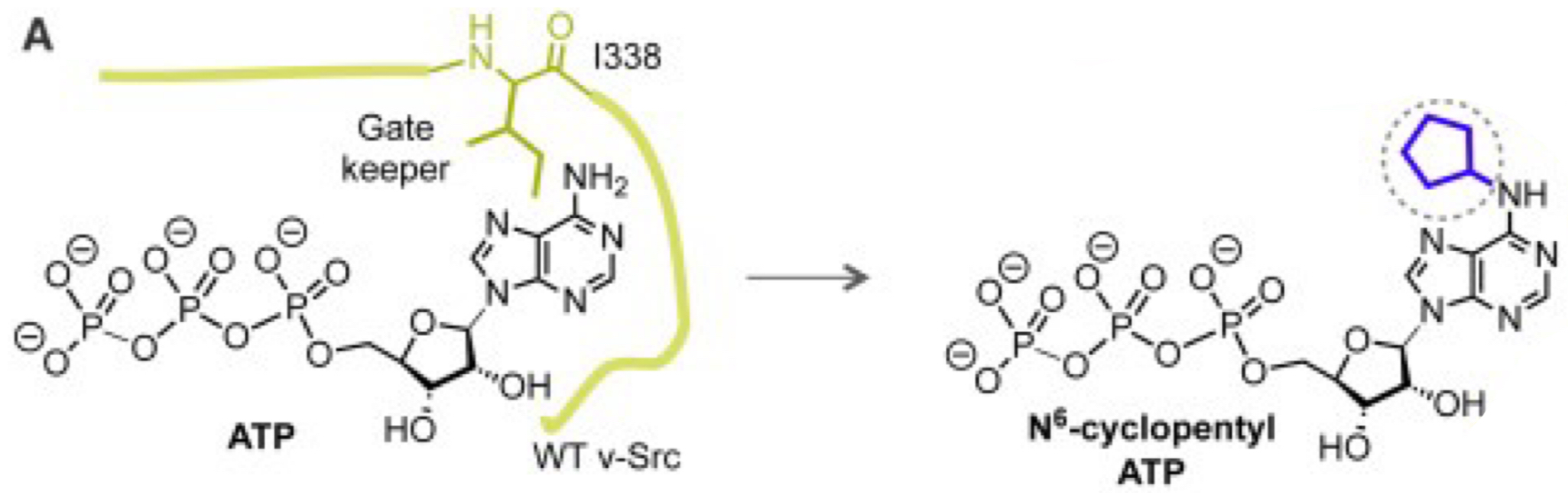
Why is it useful to engineer v-Src to use a synthetic ATP analog?
It allows scientists to study v-Src activity without interference from normal ATP, making it easier to track phosphorylation events.
What does a low Km value indicate in enzyme kinetics?
It means the enzyme has a high affinity for its substrate (binds strongly).
What is the significance of kcat/Km in enzyme kinetics?
It represents catalytic efficiency—a high value means the enzyme is very effective at converting substrate into product.
How do researchers identify v-Src substrates using mass spectrometry?
They use an engineered v-Src that incorporates radioactive 32P from the ATP analog, then detect phosphorylated proteins using MALDI mass spectrometry.
What are some v-Src target proteins identified through MALDI mass spectrometry?
Calumenin, cofilin, tubulin: proteins involved in cell structure and signalling.