Combustion of Alkanes
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
What is a combustion reaction
when a substance burns wiith oxygen to produce (heat) energy and is an exothermic (energy release) process
When a molecule burns completely in oxygen, we say it undergoes……combustion and produces ….and ……..
complete
carbon dioxide(g)
gaseous water (g) state→ water vapour
When a molecule burns INcompletely in oxygen, we say it undergoes……combustion and produces ….and ……… This type of combustion is caused when there …… or we can say……..
incomplete
2 gasses::water vapour (H2O) and carbon monoxide (CO)
(or) 1 solid: Carbon (C)→ ‘soot’ and 1 gas: water vapour (H2O)
limited amount of moles of reactant or oxygen
limiting reactant/agent
what are carbon dioxide and water vapour classed as….
greenhouse gasses
Problems associated with carbon monoxide…
toxic→ used in natural gas boilers, could be breathed in , causeing reduced amounts of oxygen into lungs and instead CO is absorbed→ could cause so muc harm to be fatal ‘toxic’
flammable: CO+O2 are very flammable reactions as reacts very easily
Problems with carbon /soot
Soot (pollutant): formed through small carbon clumps being formed as a result of hhigh temperatures in engines of old cars:
sootare subalite: easily rise up and build up in the air → eventually this build up : dirty, moky, foggy
the reuslt is called: Smog(pollution) → reduces the amount of sunlight to the ground making it harder for living things to respired eg plants
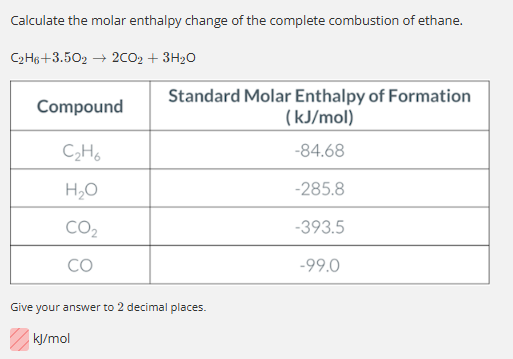
-1559.72 kJ/mol
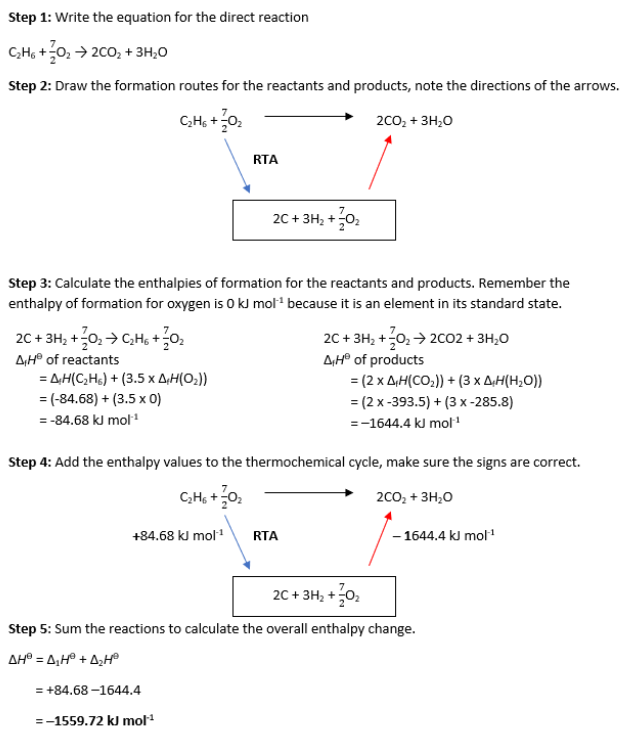
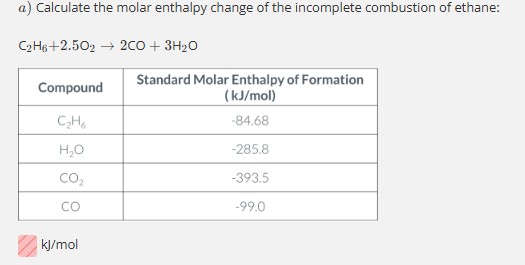
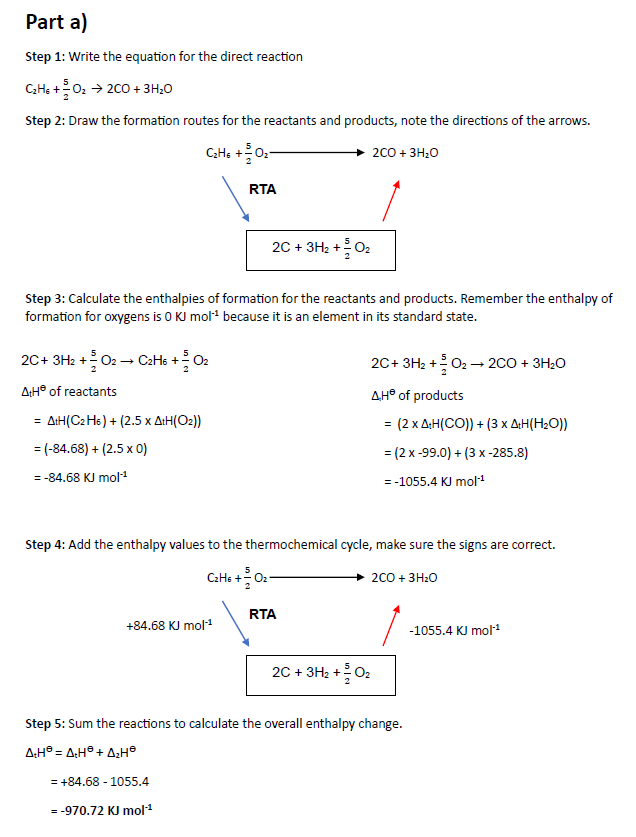
-970.72 kJ/mol
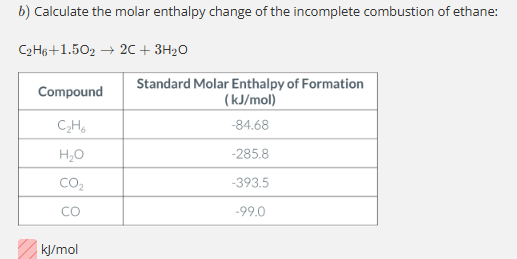
-772.72 kJ/mol
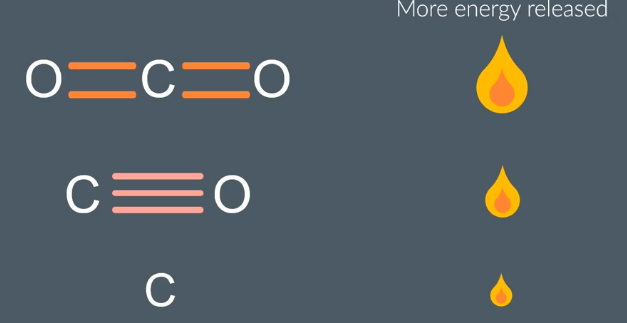
How does complete combustion produce more nergy than incomplete combustion
makingbonds is an exothermic/energy relaseing process
∴ with complete producinf CO2makes 4 bonds relaseing more energy than the products from incoomplete
we dont accound for H2o number of bonds as this is formed regardless of which type of combbustion
Why is omplete combustion less expensive
bcs we need to burn the same amount of fuel to get more energy
what organic compounds do the impurities form in the alkane eg) CH4S form in complete combustion ……
give the mollecule and state of what these would form : S, N, Si
CH4S + 3O2→ CO2+2H2O+SO2(g)
SO2(g)
NO(g)
SiO2(s)
id teh equaton doesnt spedify what type oof combustion to use…
automatically assumecomplete combustion
Unwanted organic compounds found in fuels are called… and these make fuel /// ///// than////// as some still remain in the fuel
impurities
less efficient
compleye combustion of alkenes
why is sulphur dioxide a pollutant
causes respurutaroy issues/,akes it harder to breathe in high concentrations
So2(g) + H2O(g) → H2SO4 (l) ∴ when sulphur dioxide reacts with oxygen produces sulphuric acid→ ‘acid rain’
why is acid rain bad
↑ acidicty in soil making it harder frocrops to grow
↑ acidicty in rivers/ponds ect which denature bacteria and kill wildlife by making habitat difficult to live in