biological molecules
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Why is water a polar molecule
Oxygen has a small negative charge and hydrogen has a small positive charge
What is a hydrogen bond
Water molecules are attracted to each other due to being polar molecules
What are the six properties of water
high specific heat capacity
Ice less dense than water
High latent heat of vapourisation
Cohesion
Metabolite
Why is water having a high specific heat capacity beneficial
Lots of heat energy goes into breaking bonds rather than changing temperature so water acts as a buffer so it can act as a habitat
Why is ice being less dense than water beneficial
It provides a habitat and insulates the water below and keep it from freezing
Why is having a high latent heat of vaporisation beneficial
A large amount of heat needed to evaporate water which allows organisms to cool without much water
Why is water being a solvent beneficial
For chemical reactions and to transport substances as they easily dissolve via blood plasma or xylem tubes
Why is cohesion beneficial
It is caused by hydrogen bonds and it allows water to travel up narrow tubes and causes surface tension to allow small organisms to survive on surface
Why is water being a metabolite beneficial
It’s essential for metabolic reactions such as respiration and photosynthesis
What do all carbohydrates consist of
Carbon, hydrogen and oxygen e.g. C6H12O6
Wat is glucose since it contains 6 carbons
A heroes sugar
What is the general formula for monosaccharides
(CH2O)n where n is 3-7
What is the diagram for glucose
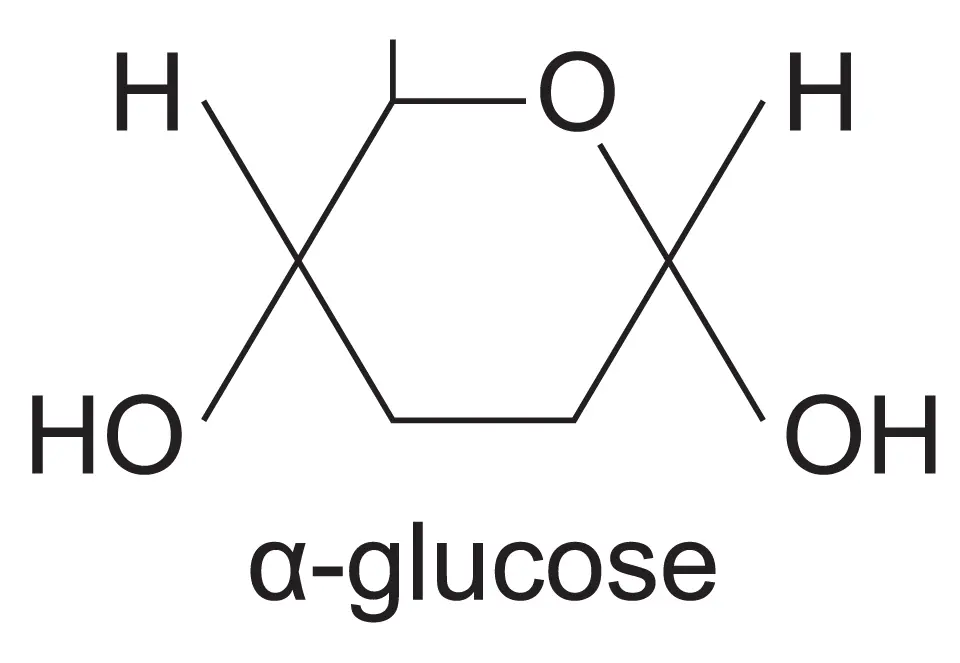
What are the three examples of monosaccharides
Glucose, fructose and galactose
What is a property of monosaccharides
They are soluble in water as they have a large number of hydroxyl groups which can form hydrogen bonds with water molecules so they are hydrophilic
What are the two isomers of glucose
Alpha and beta glucose
What is the structure of beta glucose
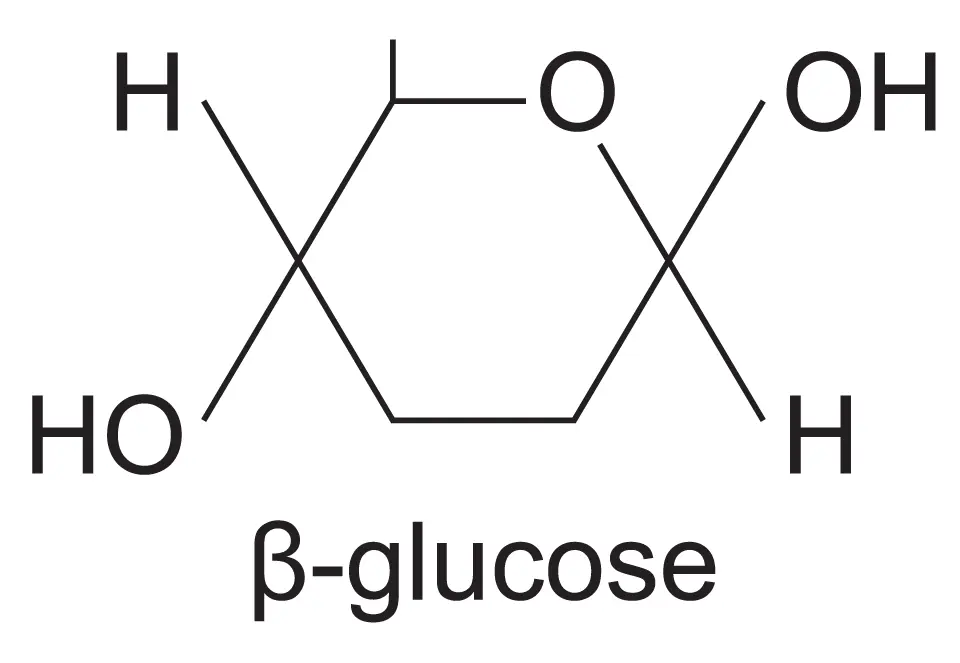
What is the difference between alpha and beta glucose
In alpha glucose, the OH group is below the carbon-1 but in beta glucose, the OH group is above the carbon 1 (ABBA)
What forms a disaccharide
When two monosaccharides chemically react together
Examples of disaccharides
Glucose + glucose = maltose
Glucose + galactose = lactose
Glucose + fructose = sucrose
What happens when two glucose form maltose
A condensation reaction takes place as water is produced from a hydrogen atom from one and hydroxyl group from another and a glycosidic bond is formed
What is a hydrolysis reaction
Adding water to disaccharides breaks the glycosidic bond, converting them back to monosaccharides
What is starch
A storage polysaccharide found in plants
Why is storing glucose in its form hard
It’s extremely soluble due to the hydroxyl groups as it is polar so they can form hydrogen bonds with water so it makes water move into cells by osmosis
How does starch fix glucose inability to store problem
It is insoluble
What are the two types of starch
Amylose and amylopectin
What is amylose
Polymer of a-glucose and it twists into a compact helix with hydrogen bonds forming along glucose molecules
What is amylopectin
Polymer of a-glucose with a branch every 25-39 glucose connecting to another glucose chain via a 1-6 glycosidic bond
Why does amylopectins structure benefit it
It is heavily branched so has lots of ends so it leads to a faster breakdown via amylase which breaks down starch by acting at the ends of molecules
How is starch broken down back into glucose
By water via a hydrolysis reaction which breaks the glycosidic bond
What are the properties of starch (3)
compact = large amount of glucose stored
Insoluble = doesn’t cause water to enter cells
Polymer = too large to diffuse out
What is glycogen
Storage polysaccharide in animals found in liver and muscle cells
What is glycogens structure
Polymer of a-glucose arranged like amylopectin but more branched so is more compact
Why must glycogen have a lot of branches
To convert back to glucose rapidly which is essential for animals due to a high rate of respiration
What are the properties of glycogen
highly branched
Insoluble
Large
What is cellulose
Structural polysaccharide found in cell wal
What is celluloses structure
Polymer of b-glucose but since the hydroxyl groups is above carbon 1, every 2nd carbon flips so it can still form a 1-4 glycosidic bond
How are cellulose fibres formed
Since cellulose is a straight chain, hydrogen bonds form with neighbouring chains which are strong due to the sheer amount which form micro fibrils which group to form microfibrils then fibres
What is the features of the cellulose cell wall
strong so provides structural support
Permeable so molecules can enter such as water which causes the cell content to be pushed outwards, preventing it from bursting