
Looks like no one added any tags here yet for you.
Define Glycolysis
A 10-step anaerobic metabolic pathway that metabolizes 1 molecule of glucose into 2 molecules of pyruvate.
The net gain is 2 ATP and 2 NADH. It occurs in the cytoplasm and is the first step in carbohydrate metabolism, providing energy for cellular processes.
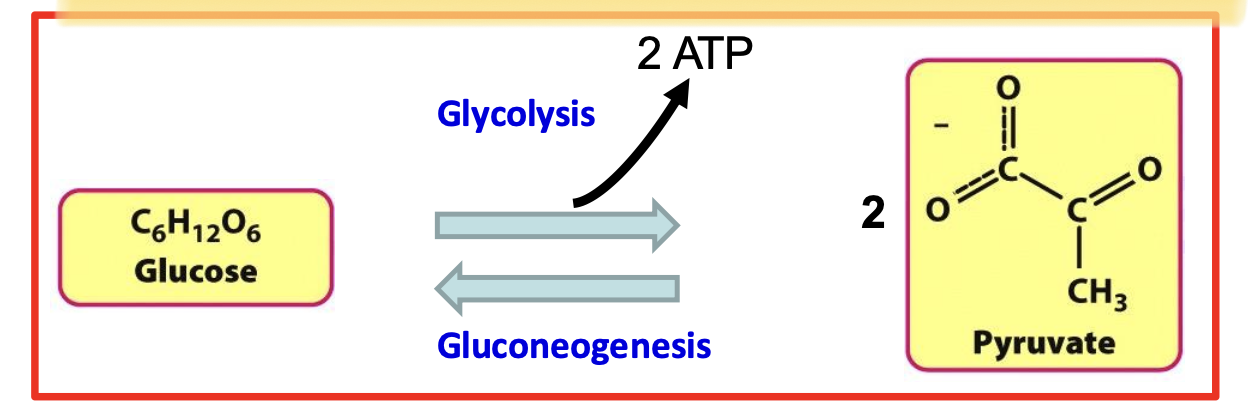
Glycolysis Net Reaction
Glucose + 2 ADP + 2 Pᵢ + 2 NAD⁺ → 2 Pyruvate + 2 ATP + 2 NADH + 2 H⁺ + 2 H₂O
Committed step
The first irreversible enzymic reaction in a metabolic pathway, usually controlled by an allosteric enzyme.
Why is glucose a universal fuel?
It is used exclusively by the brain (under normal conditions) and by red blood cells.
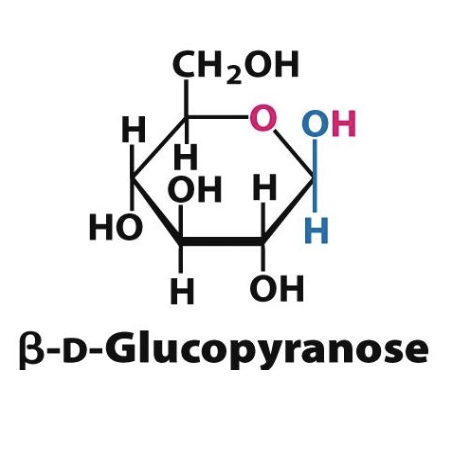
Glucose’s stability and low reactivity in its ring form (ß-D-glucose, with all OH in equatorial) make it a preferred fuel in biological systems.
Stages of glycolysis
Stage 1 - Trapping and Preparation (1-5): glucose is trapped in the cell, converted into 2 3C molecules that are easily interconverted. Consumes 2 ATP
Stage 2 - Payoff (6-10): 3C molecules GAP are oxidized to pyruvate. Happens twice. Generates 2 NADH, 2 ATP
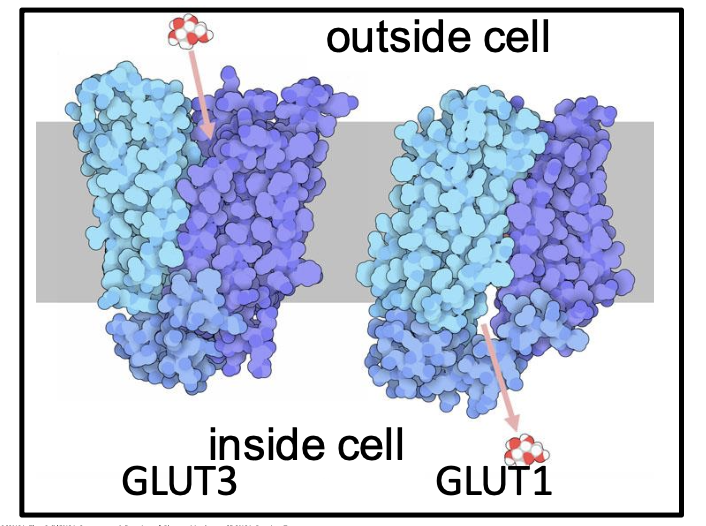
GLUT transporters
Tissue-specific transporters that facilitate the passive transport of glucose across the cell membrane from [high glucose] to [low glucose]
GLUT1 and GLUT3 support basal glucose uptake in all mammalian tissue (continuous transport at a given rate, Km = 1 mM)
GLUT2 ensures that glucose enters the liver/pancreatic ß-cells when [C] is high (due to high Km=15-20 mM)
GLUT4 increases with insulin and endurance training (muscle and fat cells)
GLUT5 is a fructose transporter in the SI
What is glucose generated from?
Dietary carbohydrates glycogen and starch.
→ 𝛼-1,4 carbohydrate bonds digested by 𝛼-amylase: maltose + maltotriose which yields glucose by enzyme maltase
→ lactase digests lactose: glucose + galactose
→ trehalase digests trehalose = 2 glucose molecules
→ sucrase digests sucrose: fructose + glucose
Structure of glucose transporters
→ 12-transmembrane-helix structure, with hydrophobic residues exposed to membrane surface
→ polar channel that, upon binding glucose, changes shape on the opposite end allowing exit
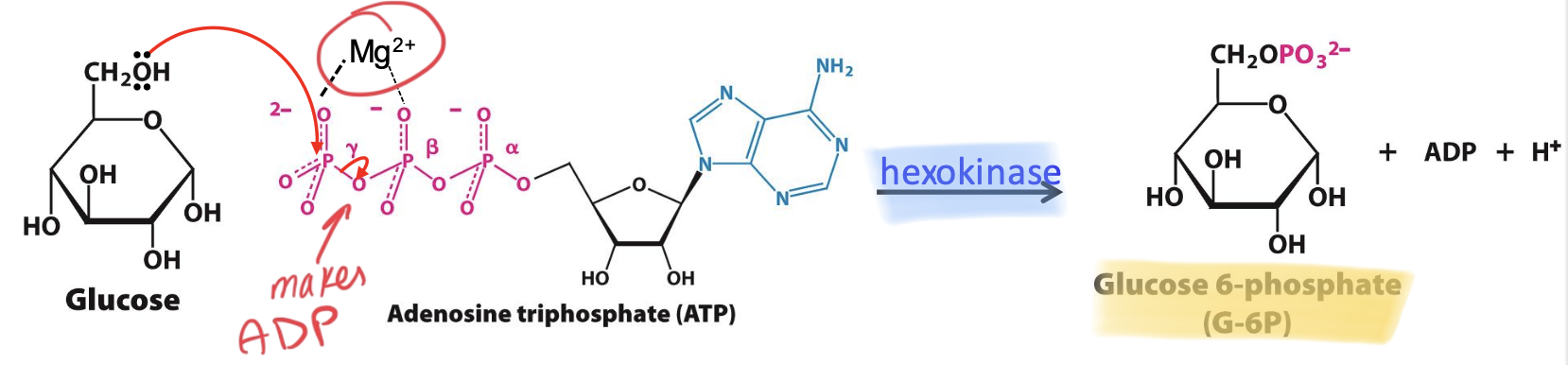
Hexokinase
Enzyme that catalyzes the 1st step (along with Mg²⁺ cofactor) : Glucose → Glucose-6P.
→ transfers a phosphate group from ATP to glucose = traps glucose in cell (too polar to pass thru membrane transporter)
→ Induced fit to enhance specificity & prevent side rxs: conformational change upon binding Glu brings active sites closer, excluding H₂O
Step 1
Glucose + ATP → Glucose-6-phosphate + ADP + H⁺
C6 OH⁻ on glucose attacks 𝛾-P of ATP. Cofactor Mg²⁺ facilitates the process by shielding -ve charges. Lowers intracellular [Glu] to allow more uptake IRREVERSIBLE, 1 ATP consumed.
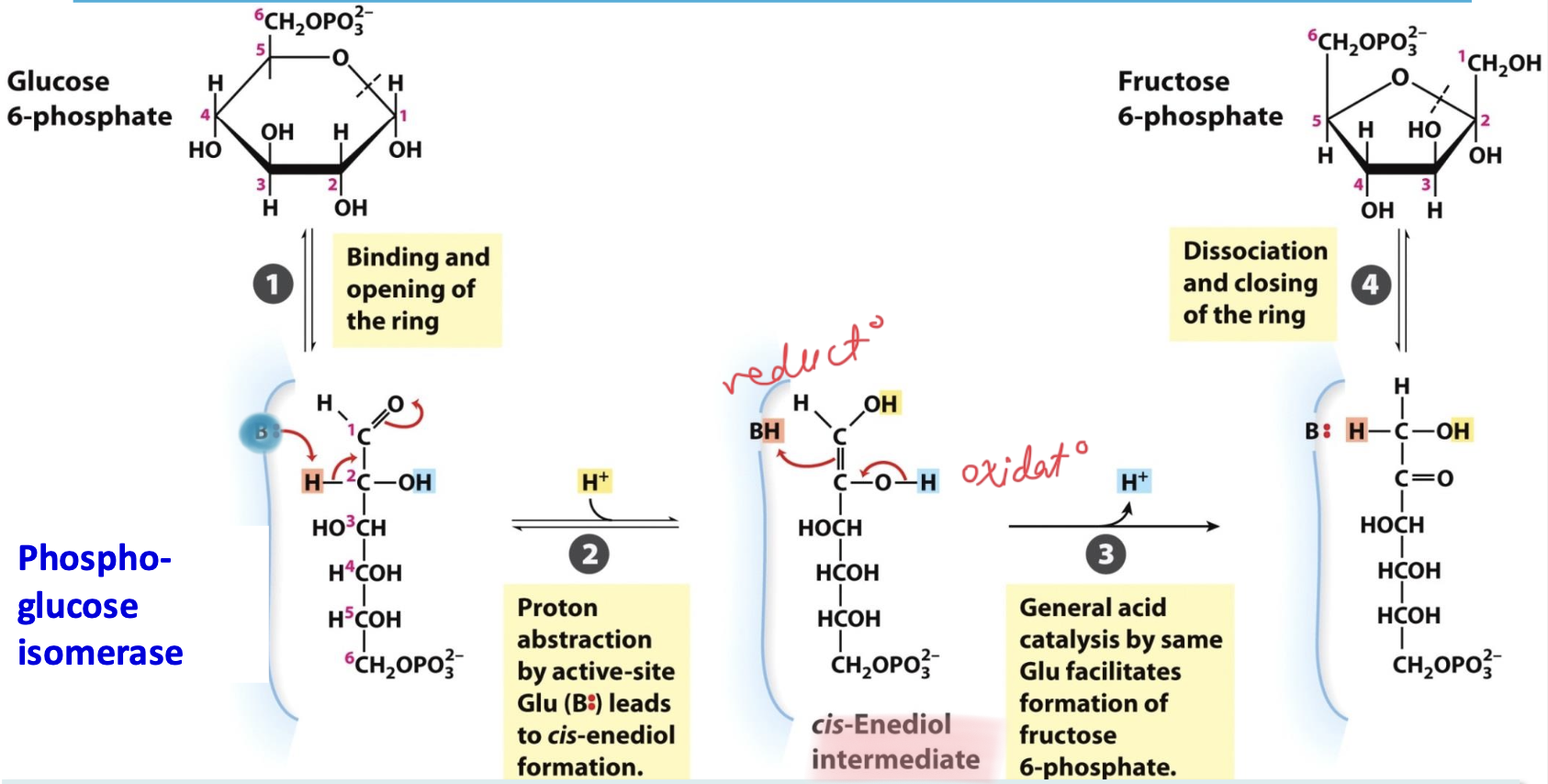
Phosphoglucose isomerase
Enzyme that catalyzes step 2 (isomerization): Glucose-6P → Fructose-6P
→ catalyzes the isomerization of aldose to ketose
→ opens G-6P ring and promotes formation of F-6P ring
Step 2
Glucose-6-phosphate → Fructose-6-phosphate
After ring opening, C2 proton is extracted by Base (Glu) = enediol intermediate. Acid catalysis of protonated Glu generates ketose F-6P. This oxido-redox is REVERSIBLE
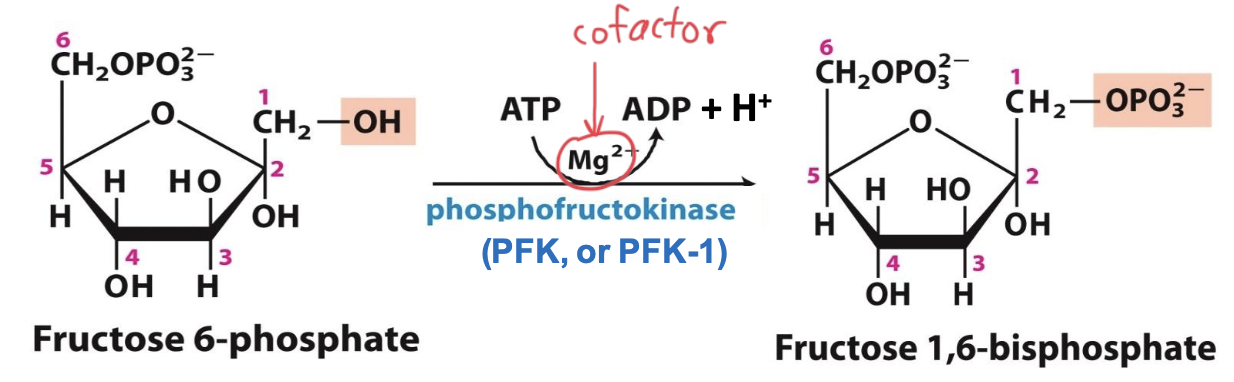
Phosphofructokinase (PFK-1)
Enzyme that catalyzes step 3 (along with Mg²⁺ cofactor): Fructose-6P → Fructose-1,6-BP
→ allosteric enzyme that regulates the pace for glycolysis/metabolism
→ tetramer w/ 4 catalytic sites. AMP & ATP are allosteric regulators that bind distinct regulatory sites.
→ phosphorylation @ C1 prevents G-6P reformation
Step 3
Fructose-6-phosphate + ATP → Fructose-1,6-bisphosphate + ADP + H⁺
Committed step. P of ATP transferred to C1 OH⁻. Cofactor Mg²⁺ facilitates transfer of P. The “bis” placement of Ps ensures both products can be phosphorylated. IRREVERSIBLE, 1 ATP consumed.
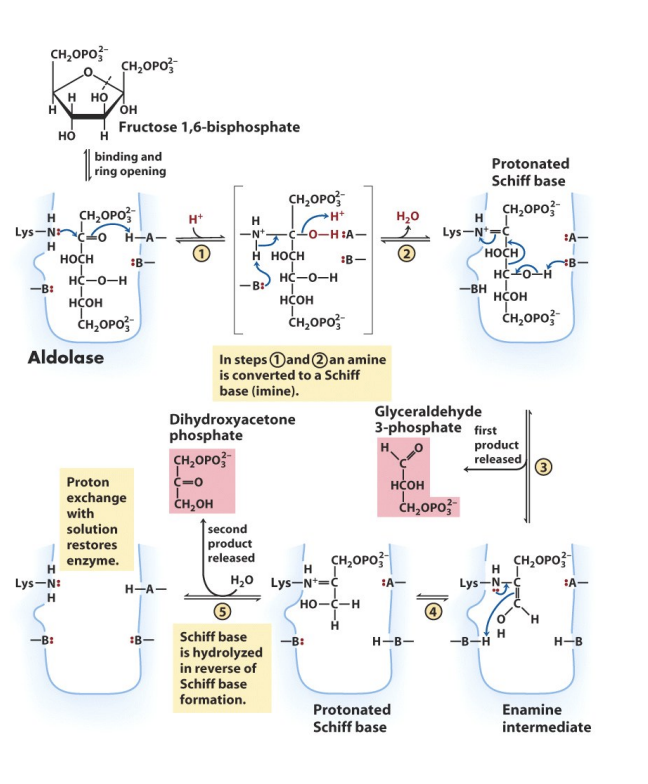
Aldolase
Enzyme that catalyzes step 4: Fructose-1,6-BP → Glyceraldehyde-3P (GAP) + Dihydroxyacetone P (DHAP)
→ cleaves open form of 6C fructose into 2 3C molecules
Step 4
F-1,6-BP → DHAP + GAP
Cleavage @ C3-C4 if C=O @ C2. Aldolase converts C=O to a Schiff base which goes thru acid/base & covalent catalysis. REVERSIBLE
Triose Phosphate Isomerase (TPI)
Enzyme that rapidly isomerizes ketose Dihydroxyacetone P (DHAP) → aldose Glyceraldehyde-3-P (GAP) during step 5 of glycolysis.
→ only GAP can continue in glycolysis, so DHAP is converted in order to maximize ATP yield
→ removal of product upon formation drives rx forward
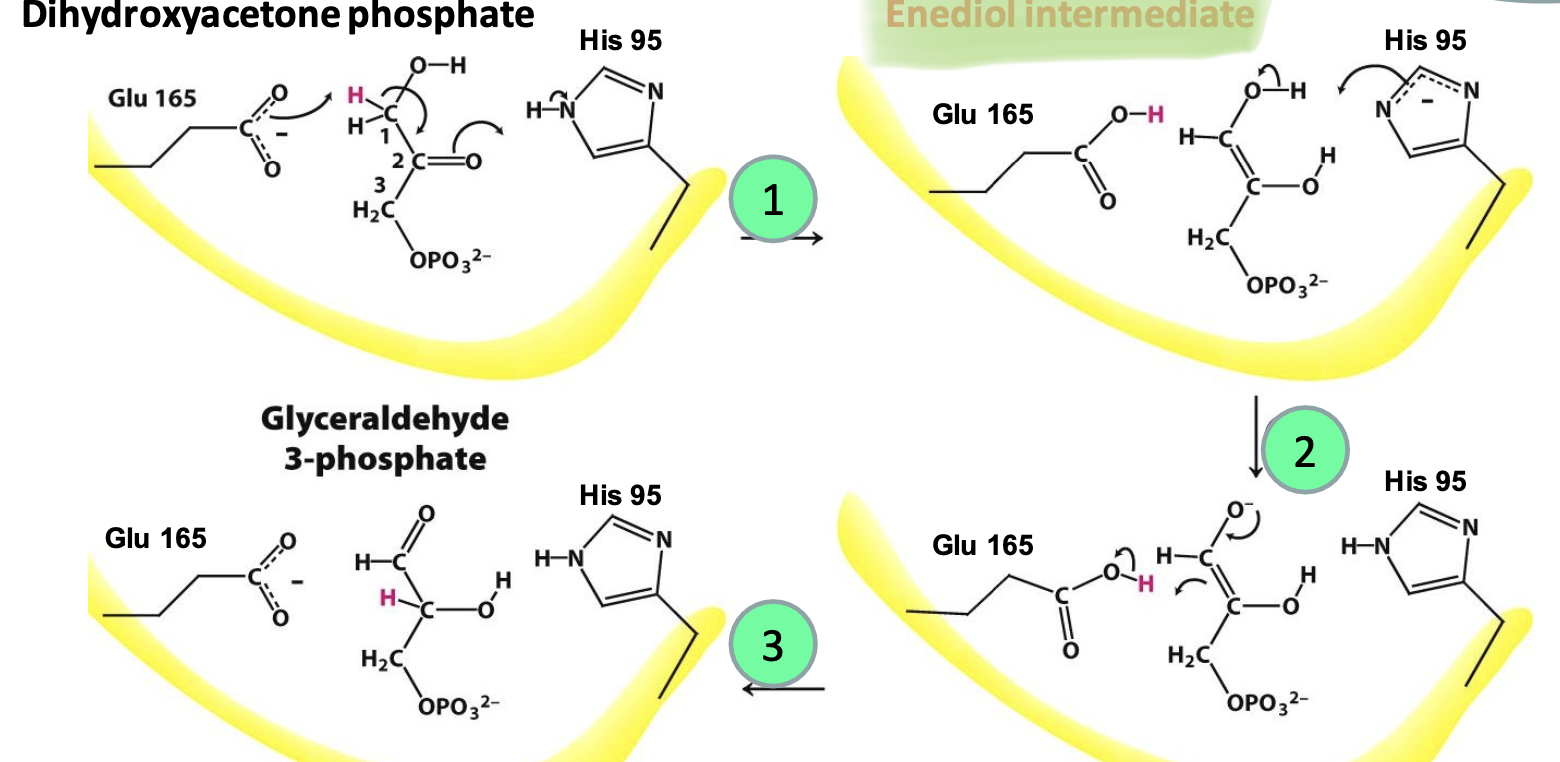
Structure of TPI
→ 𝛼𝛽-barrel (or TIM barrel) that works near diffusion limit.
→ Accelerates rx 10¹⁰ fold compared to a simple base catalyst (acetate)
→ Upon substrate binding, loop (prevents: damage, unwanted side chain rxs, enediol int. leaving) closes off active site (Glu165, His95)
Step 5
Dihydroxyacetone P → Glyceraldehyde-3P
Acid/base catalysis: Glu165 (general base catalyst) takes C1 proton while His95 stabilizes -ve charge = enediol intermediate. His95 removes H⁺ from C1’s OH. Glu165 donates H⁺ to C2, forming product. 2 GAP are formed per glucose. REVERSIBLE
Unwanted reaction formed by enediol intermediate
If the enediol intermediate leaves, it would decompose into Methyl glyoxal:
→ v quick rx (100x isomerization speed)
→ extremely reactive, would modify biosynthesis (e.g. DNA, proteins, …)
Prevented by the closing of TPI loop!
Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)
Enzyme in step 6 that oxidizes GAP to 1,3-bisphosphoglycerate (1,3-BPG). It reduces NAD⁺ to NADH incorporating an inorganic Pᵢ forming a high energy acyl-phosphate.
→ Active site: Cys149, His176, cofactor NAD⁺
→ High phosphoryl transfer potential ∆Gº’(hydrolysis) ≈ -50 kJ/mol
Step 6
Glyceraldehyde-3P + NAD⁺ + Pᵢ → 1,3-BPG + NADH + H⁺
Sum of 2 processes that use NAD⁺ as cofactor. The 1st rx (oxidation) is linked to the 2nd (acyl-phosphate formation) by thioester enzyme intermediate. REVERSIBLE
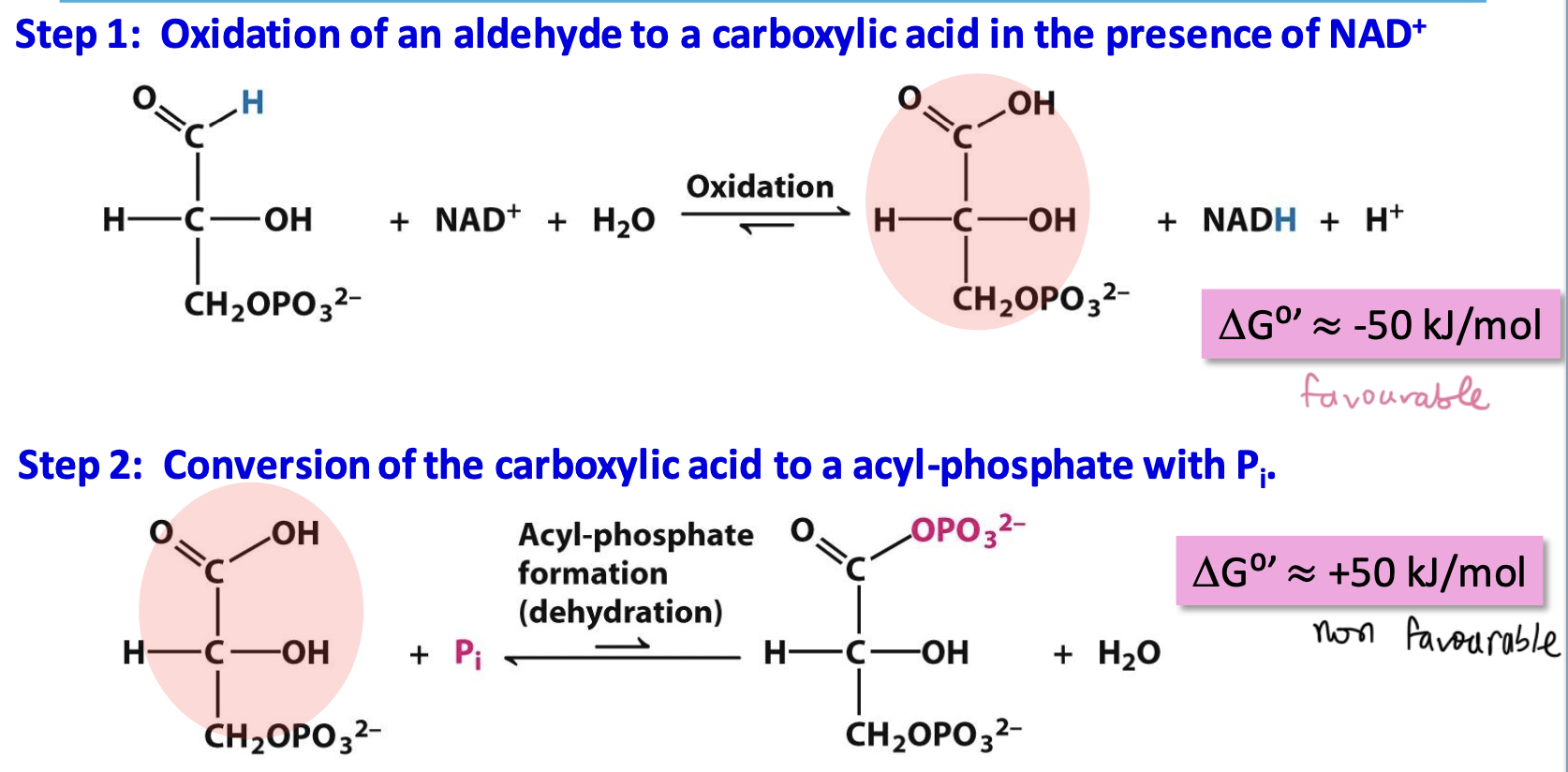
The oxidation of GAP without coupling
W/out coupling, the activation barrier of this oxidation would be to high = very slow rx
Via covalent thioester enzyme intermediate, the activation barrier is lowered since it is higher in energy than the acid
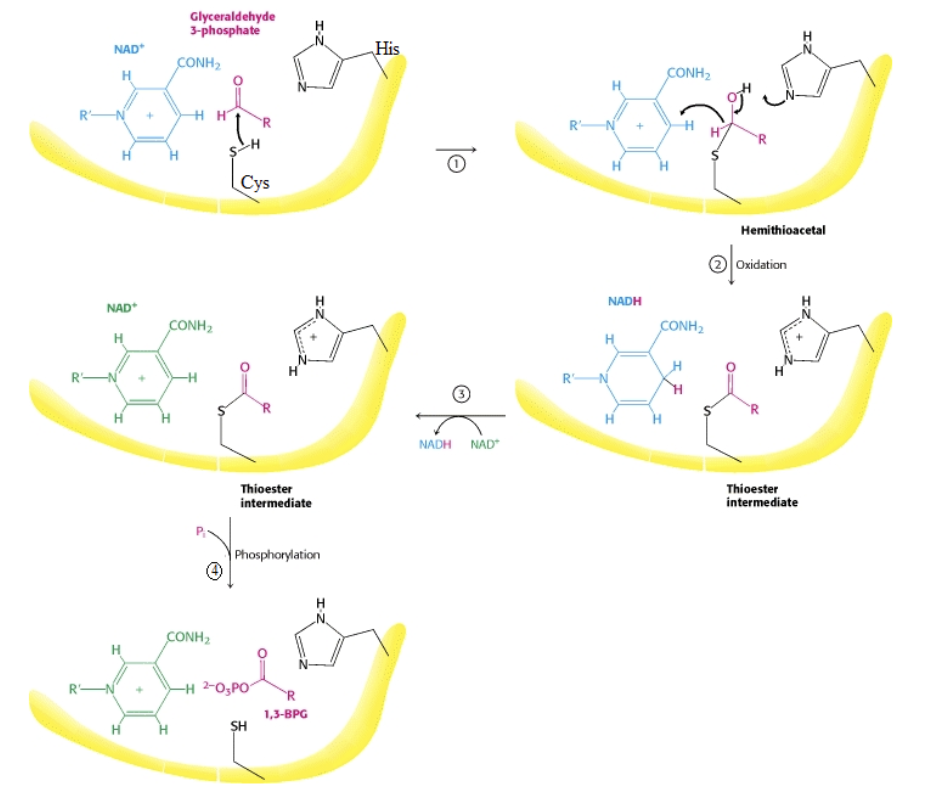
Catalytic mechanism of GAPDH
→ Cys149 and aldehyde group of GAP = hemithioacetal intermediate
→ H⁻ transferred from intermediate to NAD⁺ on enzyme = thioester int. + NADH
→ Pᵢ attacks thioester intermediate to form 1,3-BPG
Substrate level phosphorylation
Metabolic process where ATP is directly synthesized by transferring P group from a high-energy substrate directly to ADP.
→ Step 7: 1,3-BPG + ADP → 3-Phosphoglycerate + ATP by enzyme phosphoglycerate kinase
→ Step 10: Phosphoenolpyruvate (PEP) + ADP + H⁺ → pyruvate + ATP by enzyme pyruvate kinase
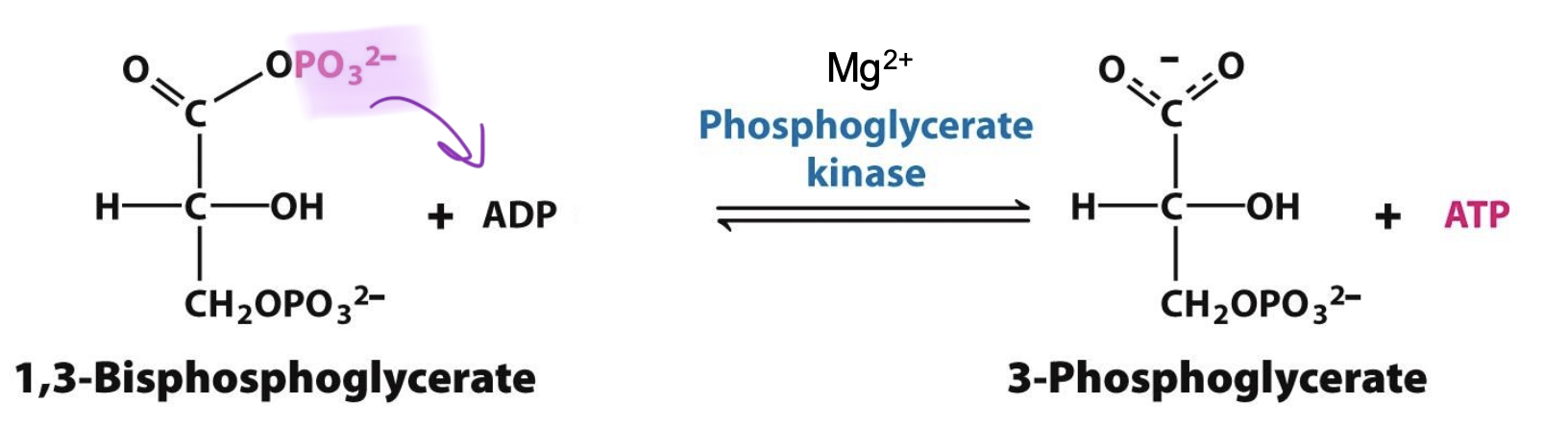
Phosphoglycerate Kinase
Catalyzes step 7 (with cofactor Mg²⁺): 1,3-BPG → 3-Phosphoglycerate
→ transfer of P group form acyl-phosphate of BPG to ADP = ATP formation
→ higher phosphoryl-transfer potential f 1,3-BPG over ATP = substrate level phosphorylation
Step 7
1,3-Bisphosphoglycerate + ADP → 3-Phosphoglycerate + ATP
The energy of 1,3-BPG (direct transfer of phosphoryl group) generates ATP = substrate level phosphorylation. Cofactor Mg²⁺ present. REVERSIBLE
→ The 2 ATP used in Stage 1 have been replenished!
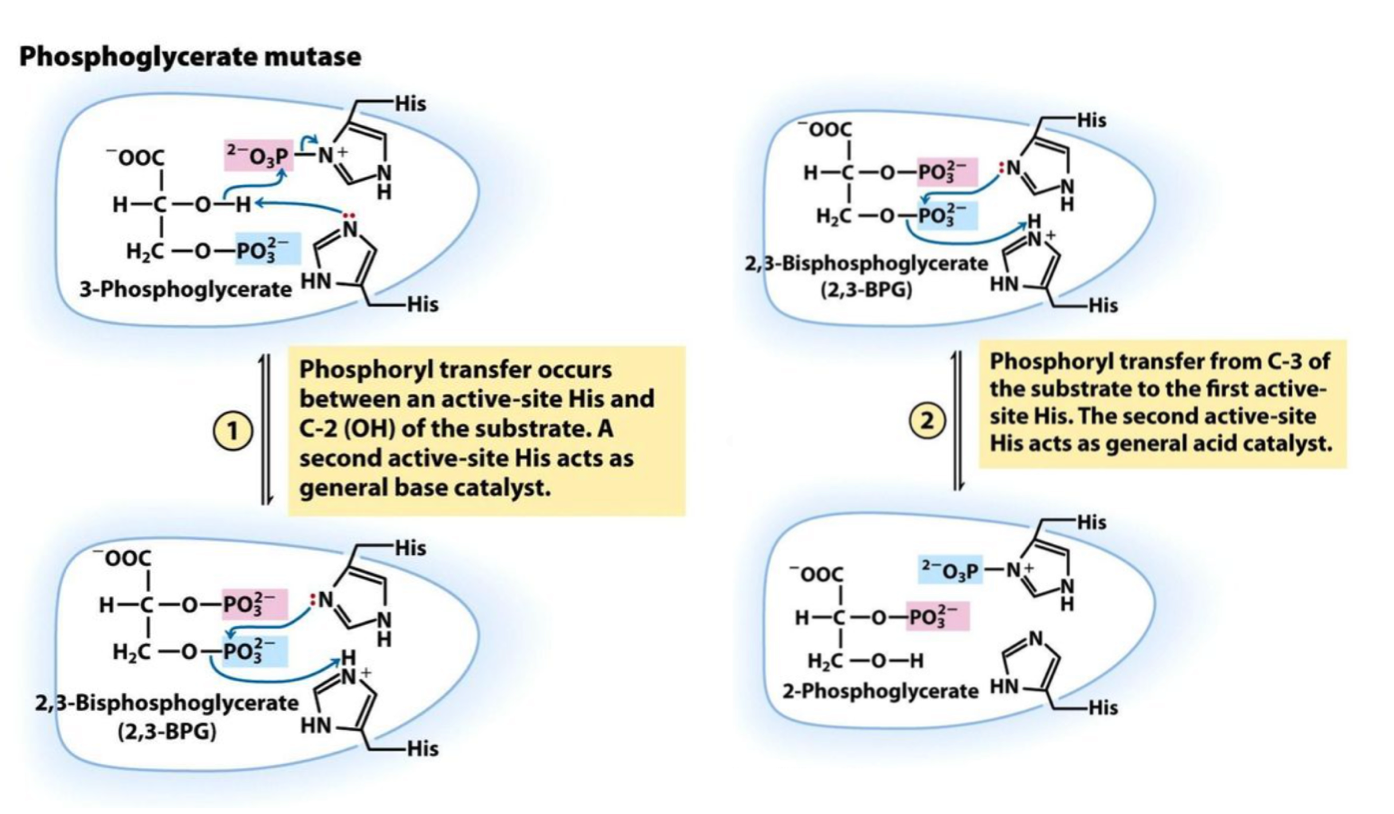
Phosphoglycerate mutase
Enzyme in step 8 that catalyzes intermolecular shift of a P group from C3 OH⁻ to C2 OH⁻: 3-Phosphoglycerate → 2-Phosphoglycerate
→ requires a catalytic amount of 2,3-BPG to keep an active site His is phosphorylated
Step 8
3-Phosphoglycerate (+ 2,3-BPG) → 2-Phosphoglycerate
Phosphorylated His₁ in active site transfers its PO₃²⁻ to C2 OH⁻. His₁ is covalently modified w/ P from C3 of now 2,3-BPG. His₂ (also in active site) acts as a acid catalyst, returning 1 and 2 to original state. REVERSIBLE
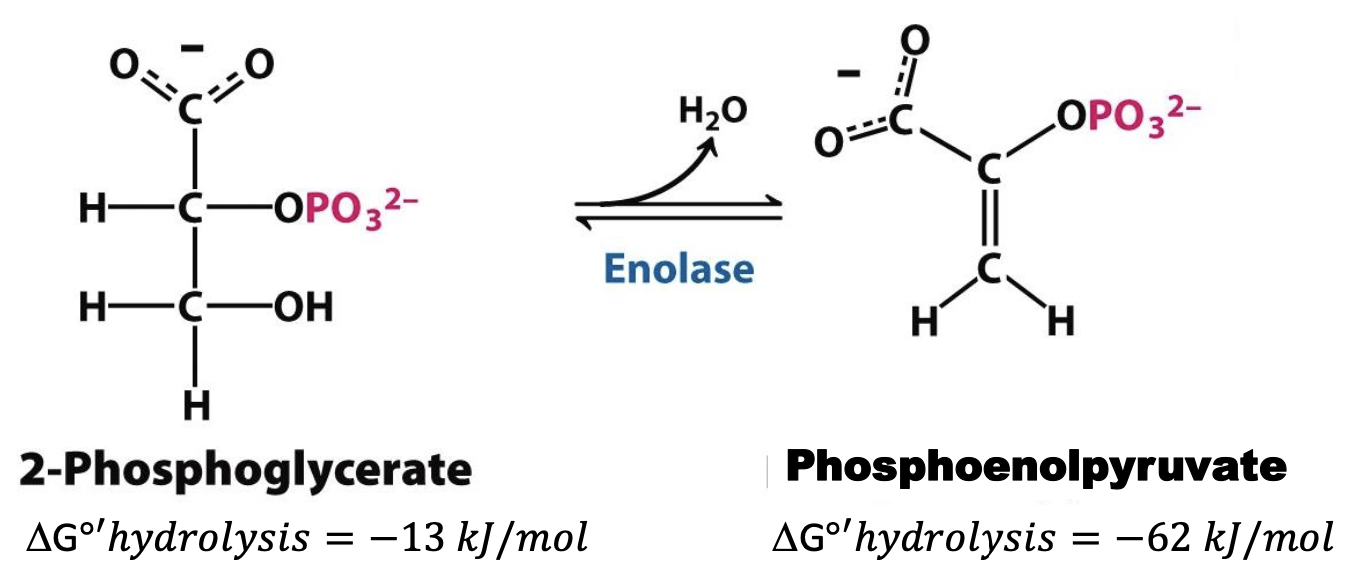
Enolase
Enzyme in step 9 that dehydrates (cofactor Mg²⁺): 2-Phosphoglycerate → Phosphoenolpyruvate (PEP)
Step 9
2-Phosphoglycerate → Phosphoenolpyruvate
H₂O molecule removed from 2-PG to generate PEP. Generates a compound with higher phosphoryl transfer potential: phosphoryl group traps molecule in unstable enol form. REVERSIBLE
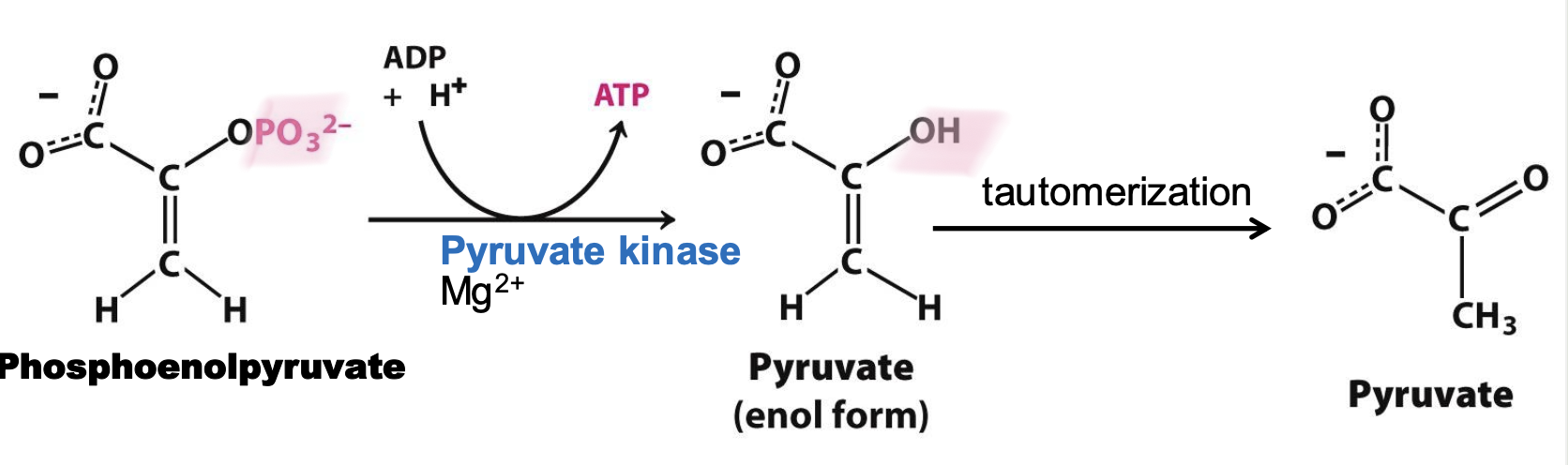
Pyruvate Kinase
Enzyme in step 10 that catalyzes the transfer of PO₃²⁻ from PEP to ATP → pyruvate in a substrate-level phosphorylation with cofactor Mg2+
Step 10
Phosphoenolpyruvate + ADP + H⁺ → Pyruvate + ATP
P group transfer to ADP = enol form of pyruvate, which is non-enzymatically converted to a stable ketone. IRREVERSIBLE, 1 ATP produced
Summary of reaction
Stage 1: 2 ATP molecules per glucose, 2 GAP produced
Stage 2: 1 ATP/GAP generated in steps 7 & 10
Net 2 ATP molecules gained, total 4 ATP produced