Principles of Chemistry I Exam 5
5.0(1)
Card Sorting
1/16
Earn XP
Description and Tags
Last updated 6:08 PM on 9/25/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
1
New cards
T or F: In a chemical reaction, particles must collide and they must collide in a specific manner.
True
2
New cards
T or F: More collisions in a chemical reaction = faster reaction.
True
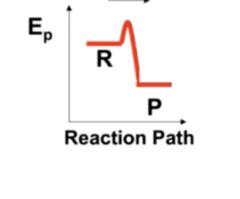
3
New cards
Endothermic or Exothermic?
Endothermic
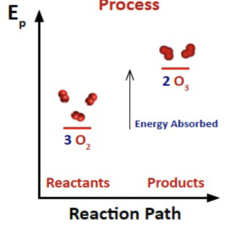
4
New cards
Endothermic or Exothermic?
Exothermic
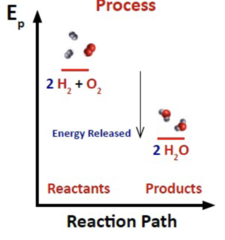
5
New cards
Endothermic or Exothermic?
Products have lower PE than reactants.
Products have lower PE than reactants.
Exothermic
6
New cards
Endothermic or Exothermic?
Products have higher PE than reactants.
Products have higher PE than reactants.
Endothermic
7
New cards
Increasing these increases the reaction rate
Temperature, pressure, # particles
8
New cards
Increasing these decreases the reaction rate
Volume, activation energy
9
New cards
Optimize these for configuration effective ness
Lowest number of gas particles, simplest structure
10
New cards
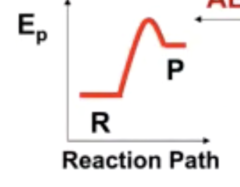
Transition state
Peak of the activation energy
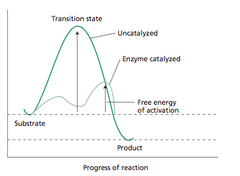
11
New cards
The catalyst does what?
Lowers activation energy
12
New cards
PF at high T
When is this preferred?
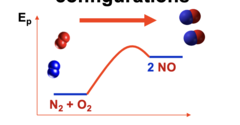
13
New cards
PF all T
When is this preferred?
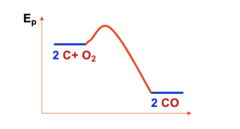
14
New cards
PF all T
When is this preferred?
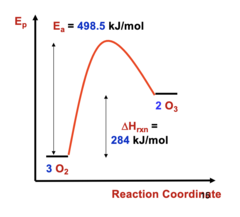
15
New cards
PF low T
When is this preferred?
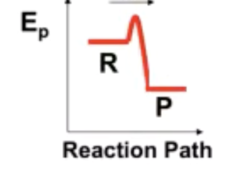
16
New cards
RF
When is this preferred?
17
New cards
PF high T
When is this preferred?