Immunology Exam 2 class 8 Basics of B cells
1/51
Earn XP
Description and Tags
whole presentation done!
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
B cell 3 points
lymphocyte derived from bone marrow, has a unique antigen receptor at surface (BCR), upon activation can give rise to plasma cells that secrete a soluble version of this receptor
how many different BCRs from the bone marrow?
10^11
what are the lymphatic and cardiovascular systems filtered by?
lymphatic: lymph nodes
cardiovascular: spleen
3 types of lymphoid structures + examples (2, 0, 2)
primary lymphoid organs (thymus, bone marrow), lymph nodes and spleen, mucosal associated lymphoid tissue (eg peyers patches, tonsils, etc.)
places that lymphoid cells go (5)
blood lymphocyte pool, lymph nodes, tertiary extra-lymphoid tissue (eg skin), spleen, bone marrow
lymphocyte recirculation (4 places)
goes between lymph nodes and then through blood, to inflamed tissue, and back through lymphatic system
two parts of the spleen
white and red pulp- white pulp has white blood cells
Are T and B cells in the same part of the lymph node?
no, B cells more on periphery (follicle), T cells more in center (in paracortex)
two ways to look at structure of antibodies
structurally - how proteins are connected
functionally - variable and constant region
how is even more variability added to BCR
joint diversification by insertion of random nucleotides
somatic hypermutation
random DNA mutations in the binding site (variable region) of the BCR, occurs during cell division, after B cell encounters antigen, if they bind (high affinity) they are kept, otherwise they die if low affinity
what is needed for B cells to become high affinity? what happens after?
interaction with helper T cells, then can differentiate into memory or plasma B cells
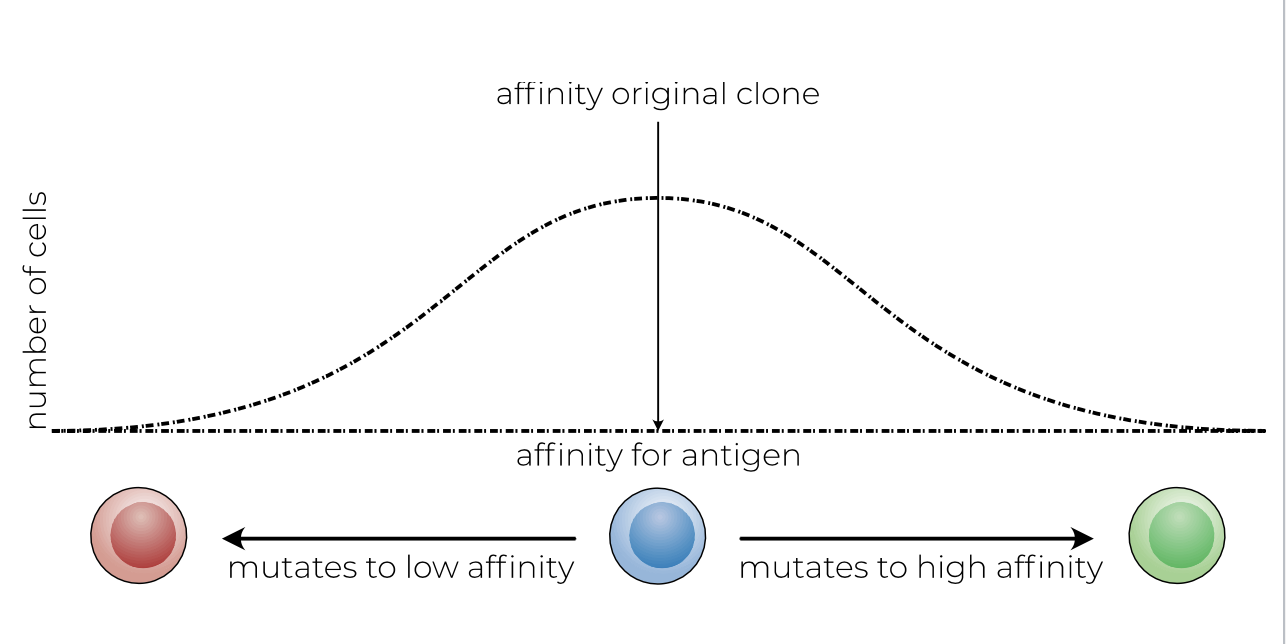
what does this image show
somatic hypermutation
what happens with germinal center B cell with mutated low-affinity surface Ig
BCR is not cross-linked and B cell cannot present antigen to T cells, B cell dies by apoptosis
what happens in lymph nodes for B cells to get activated? (3 steps)
DC with antigen migrates to paracortex, activates T cells including helper T cells, those more to the follicle and activate B cell
2 phases of antibody production (3 points each)
1st: Fast (within 2 days- life saving), complexing of antigen (neutralization) and complement activation, relatively low affinity
2nd: Slow (7-14 days), selected on high affinity, dependent on Thelper cells and germinal center
Why does the constant region of Ig have to remain constant?
its where body cells/proteins bind to it, has to be recognizable
which antibody isotypes do we have (5)
IgM, IgD, IgG, IgE, IgA
3 diff kinds of antibody and which isotypes fall where
monomer (IgG (1-4), IgD, IgE)
dimer: IgA (also exists as a monomer)
pentamer: IgM
what is extra about dimer and pentamer antibodies
they contain an additional J chain between them
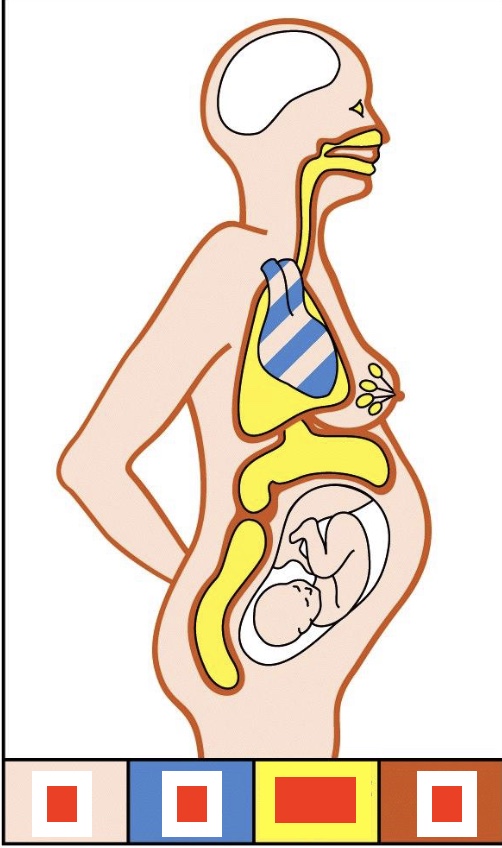
Where are what antibodies situated?
IgG (blood), IgM (heart), Dimeric IgA (mucosal surfaces), IgE (skin)

why is the brain white here?
blood brain barrier, no antibodies allowed there
why class switch between diff Ab isotypes?
each have different functions and properties
antibody parts by F domain
Fab domain- variable region (whole prong)
Fc domain/tail - constant region
How often can B cells switch Ab isotypes?
usually only once, if they switch they cannot go back
what determines isotype
differences in heavy chain
MC long
membrane-coding sequence
when does isotype switching happen and what does it affect
before somatic hypermutation, only affects constant part, does not affect variable part
How is transmembrane IgM formed? (gene level, 7 steps)
rearranged DNA, transcription, primary transcript RNA, cleavage at second poly (A) addition site and splicing, mRNA, translation/protein processing, protein
what does isotype switching cause
Ig diversity, generates Igs with distinct C regions but identical antigen-specificity (VDJ recombination)
do plasma B cells have a BCR?
no, they spit it out in the form of antibodies
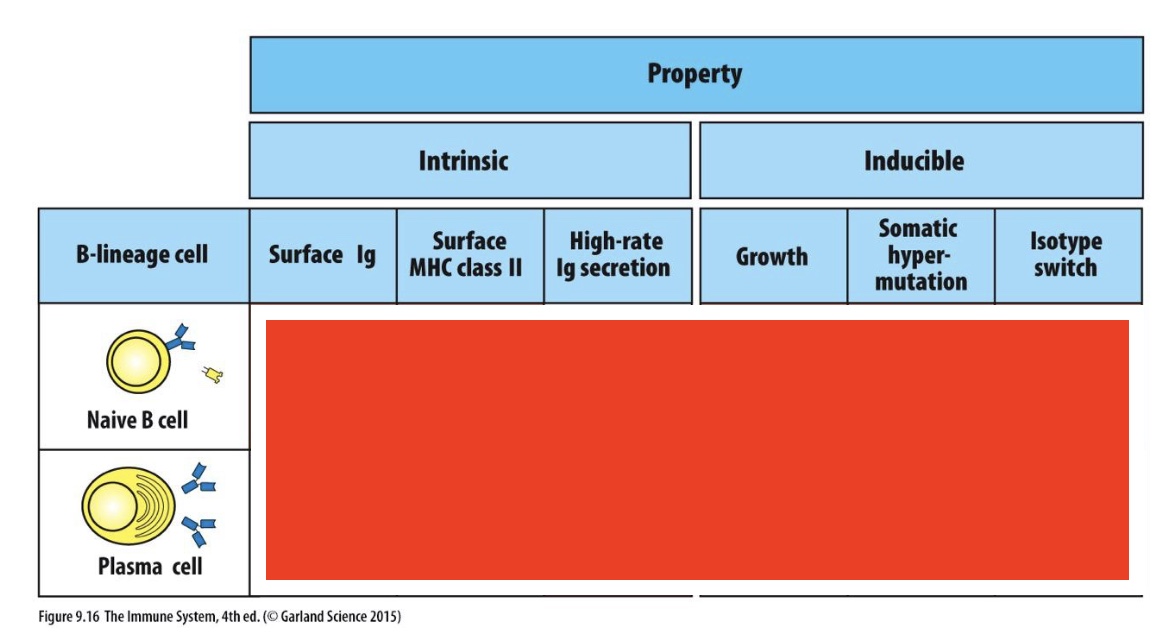
which are these
Naive B cells has yes for all, other than High-rate Ig secretion
Plasma cell has no for all other than high rate-Ig secretion (which is yes)
6 functions of antibodies
neutralization (pathogens and toxins), uptake & degradation, complement activation, antibody-dependent cellular cytotoxicity (ADCC), IgE- activate mast cells and basophils, induction of inflammation
6 functions of antibodies : how do viruses normally attack and how does neutralization of viruses work
virus binds to receptors on cell surface, receptor mediated endocytosis of virus, acidification of endosome after endocytosis triggers fusion of virus with cell and entry of viral DNA;
antibody blocks binding to virus receptor and can also block fusion event
6 functions of antibodies : how do bacteria normally attack and how does neutralization of bacteria work
bacteria colonizes human cell surface using bacterial adhesins, some species of bacteria become internalized and propagate in internal vesicles;
antibodies against adhesins block colonialization and uptake
3 examples of toxins produced by bacteria
tetanus - tetanus toxin, cholera - cholera toxin, botulism - botulinium toxin
6 functions of antibodies : how do toxins normally attack and how does neutralization of toxins work
toxins bind to celullar receptors, endocytosis of toxin:receptor complexes, dissociation of toxin to release active chain (which poisons cell);
antibody protects cell by blocking binding of toxin
6 functions of antibodies : uptake & degradation
Ab facilitate uptake of pathogens through Fc receptors bc aggregation of Ig on bacterial surface allows cross-linking of Fc receptors which causes activation of macrophage (leading to phagocytosis and degradation of bacterium)
what different types of Fc receptors do immune cells express and which ones bind which Ab?
FcγR (binds IgG) - many diff types, can be both activating and inhibiting
FcεR (binds IgE)
FcαR (binds IgA)
6 functions of antibodies : complement activation (two ways)
classical pathway, like a docking station for MACs
pentameric IgM molecule binds to antigens on bacterial surface and adopts “staple” form, C1 binds to a single IgM molecule
IgG molecules binds to antigens on bacterial surface, C1q binds to two or more IgG molecules
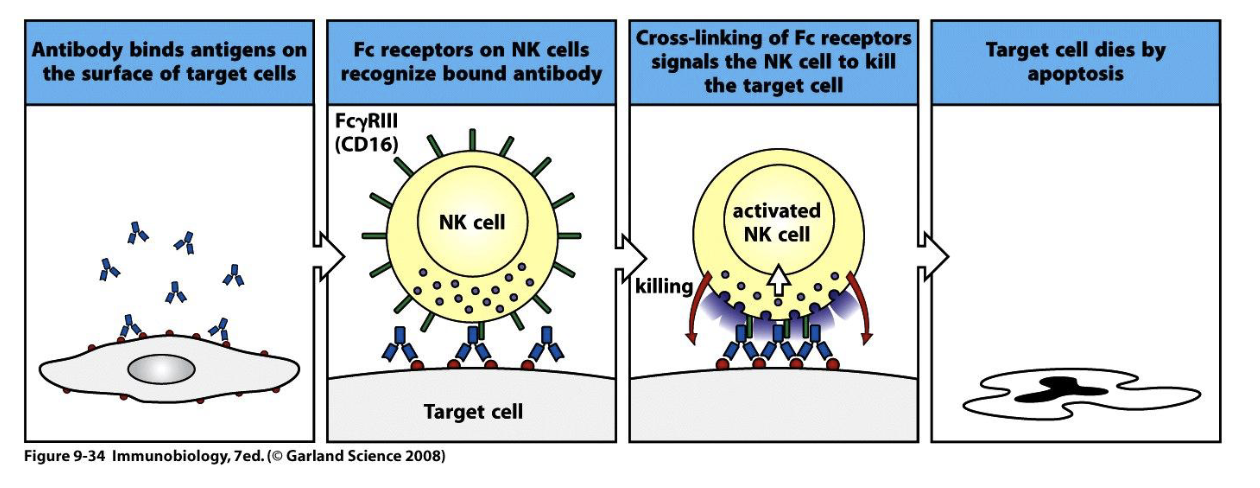
6 functions of antibodies : Antibody dependent cellular cytotoxicity (ADCC)
6 functions of antibodies : IgE can activate mast cells and basophils
resting mast cell contains granules containing histamine and other inflammatory mediators, activated mast cell: multivalent antigen cross-links bound IgE antibody, causing release of granule contents
6 functions of antibodies : antibodies can induce inflammation
based on a combination of antibodies recognized and PRRS - different cytokines are released, immune cells express different types of Fc receptors which (also?) impacts what cytokines released
Downside of antibody-induced inflammation
can backfire- in severe COVID-19 cases was actually as a result of overactive immune system rather than the virus itself, seen because disease would dramatically worsen 1-2 weeks after onset of symptoms, anti-SARS-COV-2 antibodies strongly correlated with disease severity
More detail how antibodies acted as bad guys in severely ill COVID-19 patients
COVID would lead to an early and strong rise of IgGs which lead to high titers (macrophages activated which released proinflammatory cytokines which destroyed tissue) and aberrant glycosylation (where fucose (sugar) is not present in Ab)
More detail how antibodies acted as bad guys in severely ill COVID-19 patients: 3 results
hyper-inflammation, edema, micro-thrombosis (because platelets also have Fc)
Benefits of low fucose IgG (afucosylation)
correlates with traits of protective immunity against malaria and HIV-1
features of vaccine for COVID-19
only directly against receptor binding domain (RBD) (spike protein) (almost never the current one but still provides some protection!), 2nd response much bigger (particularly clear for booster and omicron)
best neutralization for vaccine
vaccination + infection
What is different with COVID compared to flu or tetanus & consequence of that
will not create long-lived plasma cells which is why you need so many doses
Why are vaccines not completely neutralizating (esp in COVID), possible solution?
vaccine mostly gives IgG (because injected into blood) but you actually need more IgA (most infections are in the lungs) so it will not be neutralizing but will protect against severe disease, potential solution are nasal vaccines so you can produce IgA locally, has worked in monkey trials
what subclasses of Ig did the mRNA COVID vaccine produce and how was the glycosylation? Result?
IgG1>IgG2>IgG3/4, did not cause afucosylation, was okay for the body!