Gas Exchange and Transport
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Site of gas exchange
Respiratory membrane
Thickness of respiratory membrane
0.5 um
Respiratory membrane
Large total surface area for exchange → 300 million alveoli
Respiratory membrane includes
Alveolar epithelium
Basement membrane
Capillary endothelium
Fluid (air-water interface)
Both carbon dioxide and oxygen are…
which lets them pass easily through what
Lipid soluble which allows them to pass easily through both surfactant and plasma membranes
Diffusion across blood air barrier
Diffusion of gases to/from alveolar air to/from capillary plasma
Diffusion of gas across an exchange surface is proportional to…
Surface area
Pressure gradient (P1-P2)
Solubility of gas in water
Diffusion of gas across an exchange surface is inversely proportional to …
Thickness of exchange surface
Carbon dioxide and oxygen → diffusion
Carbon dioxide has a rate of diffusion 20 times greater than oxygen because it is much more soluble than oxygen
Dalton’s Law
The total pressure of a mixture of gases is equal to the sum of the partial pressures (Px) of the individual gases
Px
Partial pressure of gas x
Px = Atmospheric pressure X fractional concentration of x in the gas mixture
Px = Patm X Flx
Composition of ambient air
Nitrogen
Fraction in air % → 78
Partial pressure → 593
Oxygen
Fraction in air % → 21
Partial pressure → 159
Carbon dioxide
Fraction in air % → 0.03
Partial pressure → 0.23
Argon
Fraction in air % → 0.93
Partial pressure → 7
Water
Fraction in air % → 0
Partial pressure → 0
Henry’s Law
Amount of gas dissolved in a liquid is proportional to the partial pressure of that gas
An increase in Px = An increase amount of x in liquid
Not all gases are equally soluble in all liquids (CO2 very soluble in body fluids - N2 low solubility)
The concentration of a gas in solution is equal to its partial pressure multiplied by its solubility in the solution
Example of Henry’s Law:
Oxygen PO2 = 100 mmHg
Solubility coefficient = 0.003 mL O2/100 mL plasma/mmHg
Concentration of dissolved oxygen is 0.3 mL/100 mL plasma
Composition of tracheal air
Conducting zone: warm and humidify incoming air
Warmed to body temperature: 37 degrees C
PH2O = 47 mmHg
Px = (atm pressure - water vapor pressure) X fractional concentration of gas x
Px = (Patm - PH2O) X Flx
For carbon dioxide it is assumed that the amount of inspired CO2 is negligible
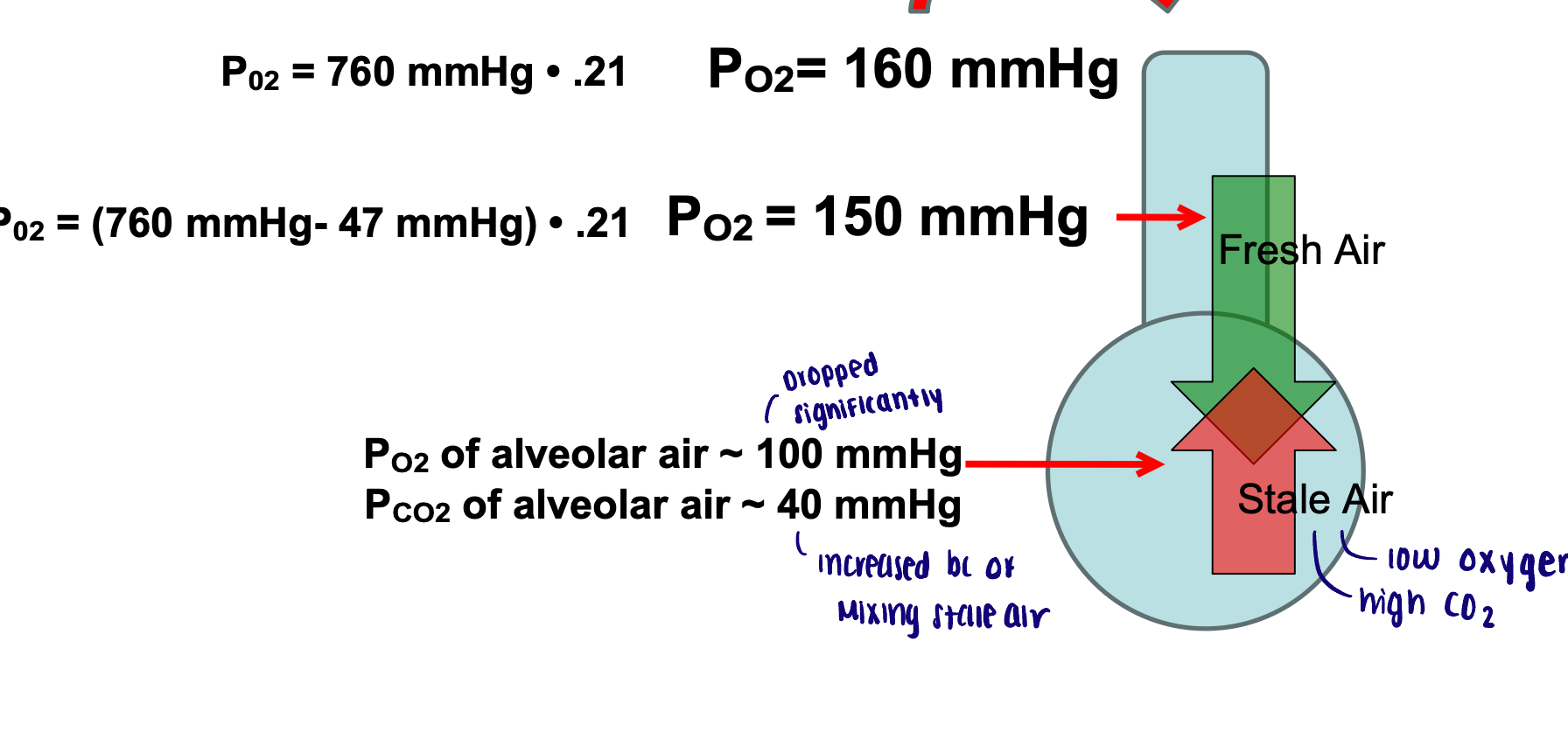
Oxygen partial pressure → composition of tracheal air
PO2 = (760 mmHg - 47 mmHg) X 0.21 = 150 mmHg
The new partial pressure decreased through humidifying
Composition of alveolar air
PO2 of alveolar air decreases and PCO2 of alveolar air increased (compared to tracheal air) due to mixing of inspired air with leftover air containing carbon dioxide
Amount of Co2 inhaled is NEGLIGIBLE
O2 → 160-150-100 mmHg
CO2 → 40 mmHg
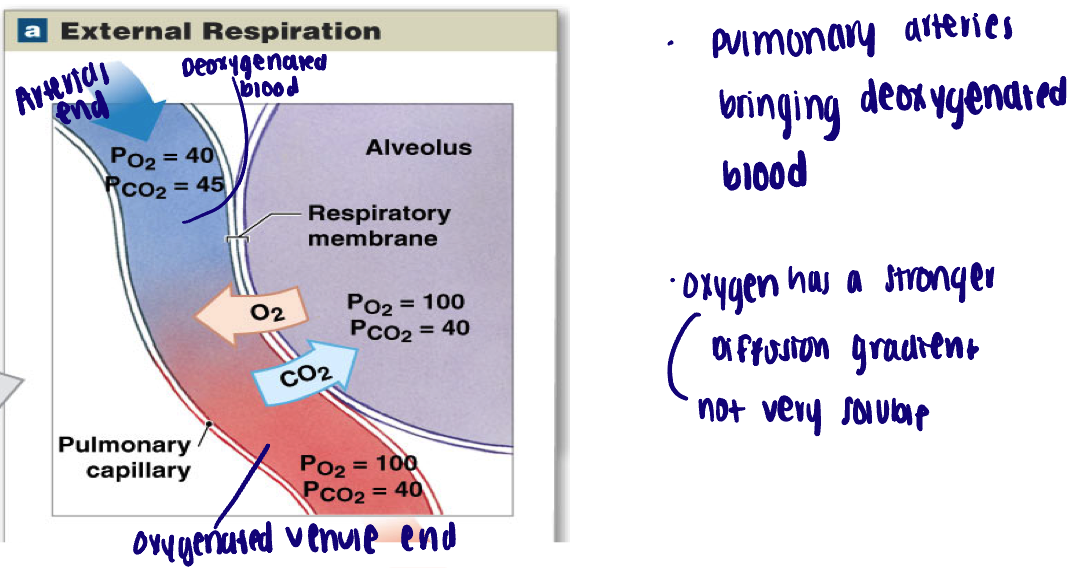
External respiration → alveoli
Exchange at respiratory membrane:
Oxygen moves down its gradient FROM alveolus into plasma
Carbon dioxide moves down its gradient FROM plasma into alveolus
Pa O2 = 40 mmHg → Pv O2 = 100 mmHg
Pa CO2 = 45 mmHg → Pv CO2 = 40 mmHg
a = arterial blood
v = venous blood
External respiration → alveoli regulation of respiration
Blood flow directed to alveoli with higher PO2 (capillaries constrict when PO2 is low)
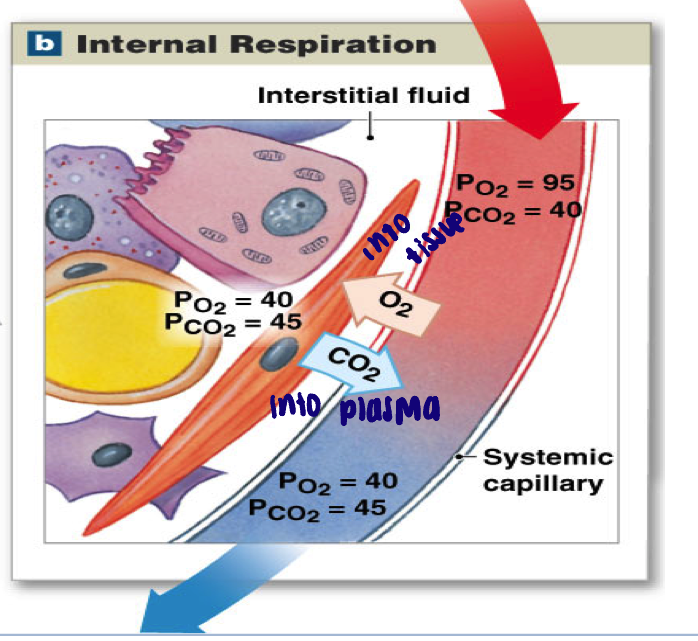
Internal respiration → tissues
Oxygenated blood delivered to tissues (internal respiration of cells consumes O2 and produces CO2)
Carbon dioxide moves down its gradient FROM tissues into blood
Oxygen moves down its gradient FROM blood into tissues
Pa O2 = 95 (basically 100) mmHg → Pv O2 = 40 mmHg
Pa CO2 = 40 mmHg → Pv CO2 = 45 mmHg
Internal respiration → tissue regulation of respiration
When tissue is active:
Interstitial PO2 falls, interstitial PCO2 rises
Increases the difference in partial pressure between tissue and arriving blood
Increases rate of diffusion
Gas transport in the blood
Diffusion moves O2 from the alveoli to plasma (liquid) and CO2 from plasma to alveoli
Limited solubility of both O2 and CO2
Peripheral tissues need more O2 and generate more CO2 than plasma alone can absorb and transport
CO2 is transported via three different mechanisms
O2 is transported primarily by RBCs (small amount in plasma)
RBCs remove gases from the plasma - diffusion continues
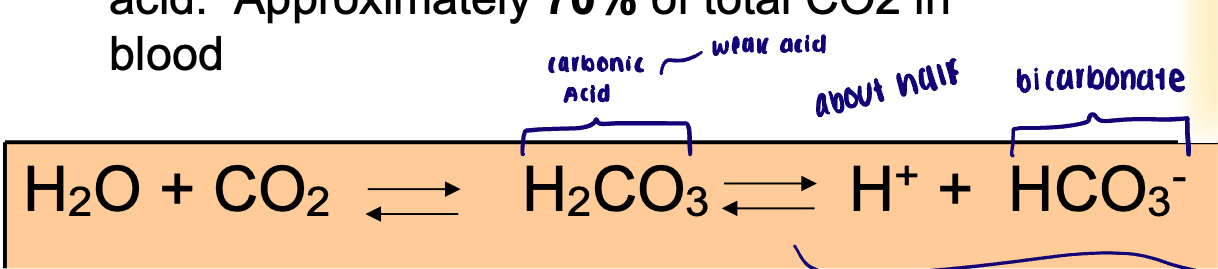
Carbon dioxide in the blood
Physically dissolved in the plasma
Approximately 7% of CO2 in blood
Bound to hemoglobin (carbamino -hemoglobin): bound to amino groups o hemoglobin molecule
Approximately 23% of CO2 in blood
Chemically modified in the form of carbon acid
Approximately 70% of total CO2 in blood
Reaction with H2O and CO2 and the enzyme
This reaction is greatly accelerated by the carbonic anhydrase (CA), an enzyme present in most cells and abundant in red blood cells
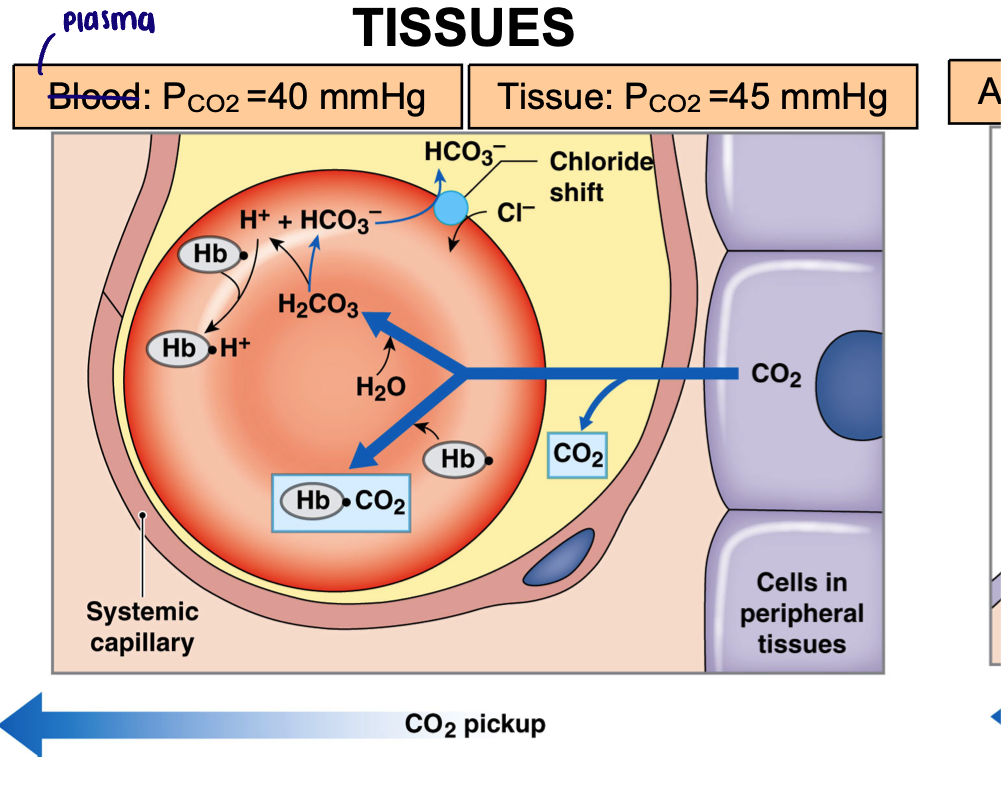
Carbon dioxide exchange in tissues
Plasma PCO2 = 40 mmHg
Tissue PCO2 = 45 mmHg
Carbon dioxide diffuses into capillary down its partial pressure gradient
Absorbed by RBCs
Carbaminohemoglobin (23%)
Converted to carbonic acid (70%)
Bicarbonate is moved into the plasma in exchange for chloride
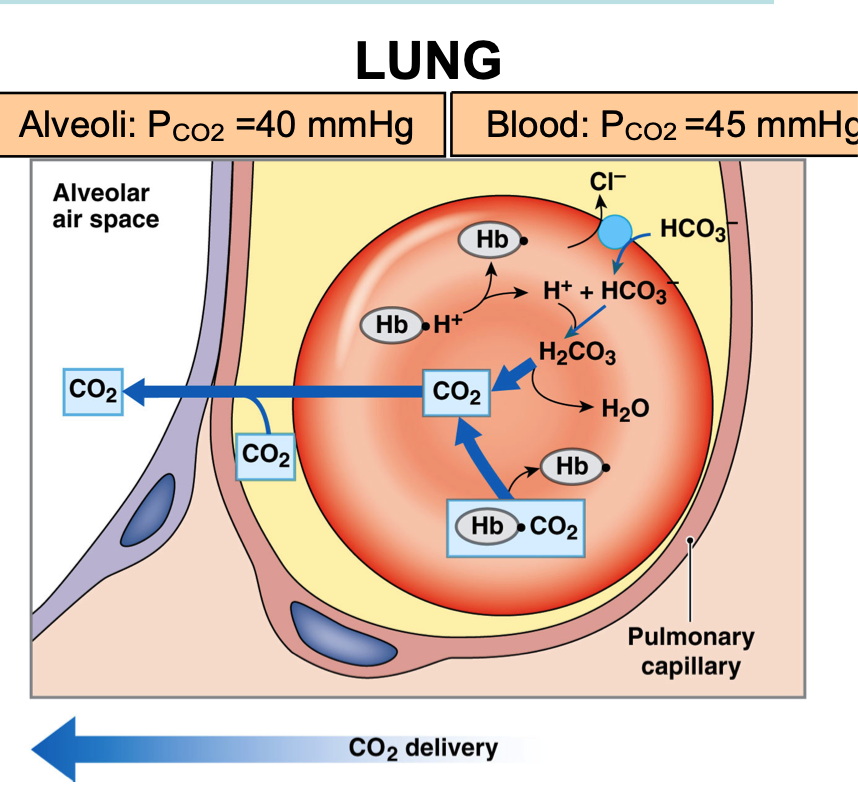
Carbon dioxide exchange in the lung
Alveoli PCO2 = 40 mmHg
Blood PCO2 = 45 mmHg
Carbon dioxide diffuses into alveolus down its partial pressure gradient from plasma
RBCs release CO2 (from two sources)
Released from hemoglobin
Bicarbonate moves into RBCs (chloride exchange)
Carbonic acid converted back into CO2
Oxygen in the blood
Physically dissolved in the plasma
Approximately 2% of total oxygen in blood
Responsible for the partial pressure of oxygen in the blood
Poor solubility in plasma - inadequate to meet body’s needs
Bound to heme group of hemoglobin
98% of total oxygen in blood
Oxyhemoglobin
Does not contribute to the partial pressure of oxygen
Each Hb molecule has 4 heme groups and therefore can carry 4 oxygen molecules
% oxygen saturation
% saturation of hemoglobin molecules with oxygen
Ex: 50% saturation → 50% of heme sites occupied with oxygen
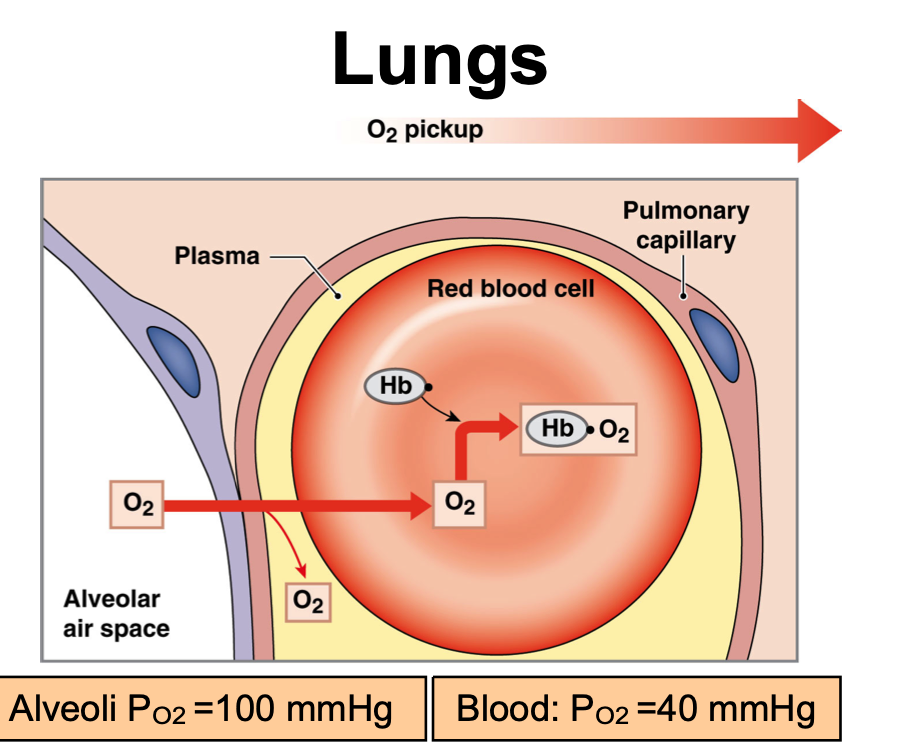
Oxygen delivery in the lungs
Alveoli PO2 = 100 mmHg
Blood PO2 = 40 mmHg
Oxygen delivery in the tissues
Blood PO2 = 100 mmHg
Tissue PO2 = 40 mmHg
Loading of oxygen on Hb helps…
Maintain the pressure gradient for oxygen (by maintaining PO2 in plasma)
Oxyhemoglobin - dissociation curve
As (plasma) PO2 rises, % saturation of hemoglobin increases
Sigmoidal shape: cooperative binding
More O2 attached, more likely for another O2 to bind
Oxygen loading onto one heme site facilitates loading of oxygen on remaining heme sites
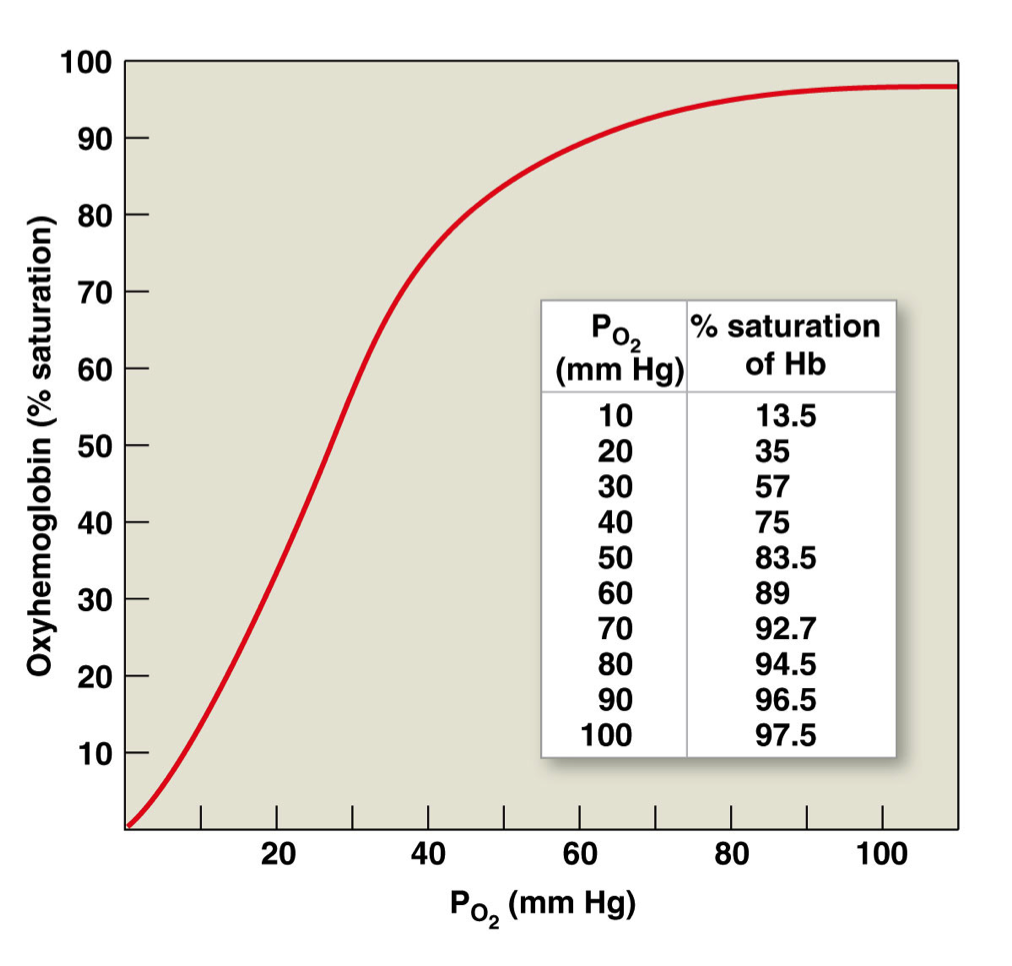
Regions of oxyhemoglobin-dissociation curve
Plateau region: oxygen loading → this is at the PO2 encountered at the lungs
Steep region: oxygen unloading → this is at a PO2 encountered at the tissues
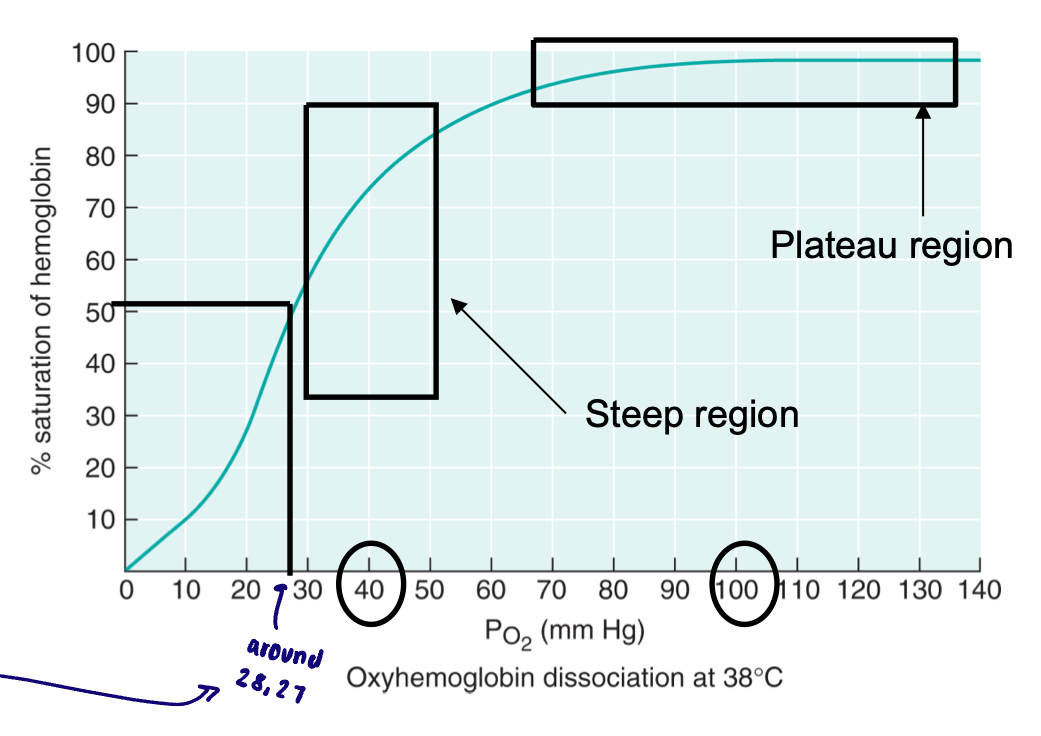
P50
Pressure at which hemoglobin molecules are half saturated with oxygen
Measure of the affinity of hemoglobin for oxygen
A higher P50 reflects…
A decrease in affinity for hemoglobin
Right shift
A lower P50 reflects…
An increase in affinity for hemoglobin
Left shift
Bohr effect
Changes in pH shifts the curve (normal blood pH: 7.4)
Increase in pH: shifts the curve to the left
P50 decreases
Affinity of O2 for hemoglobin increases
Decrease in pH: shifts the curve to the right
P50 increases
Affinity of O2 for hemoglobin decreases
Increased CO2 will increase H+ and therefore lower pH which shifts curve. H+ binds to hemoglobin which decreases the affinity of hemoglobin for oxygen
Increased CO2 will also shift curve to right independent of pH change. Increased formation of carbaminohemoglobin which decreases affinity of hemoglobin for oxygen
Temperature - oxygen-hemoglobin curve
Increases in temperature shifts curve to the right
Decreases in temperature shifts curve to the left
Carbon monoxide
Outcompetes oxygen for the heme binding sites of hemoglobin
250 times greater affinity for hemoglobin than oxygen
Carbon monoxide also shifts the oxyhemoglobin dissociation curve to the left
Heme groups not bound to CO will have an increased affinity for oxygen and will not give up oxygen as easily to the tissues