haemoglobin
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
OXYGEN TRANSPORT IN THE BODY
Dissolved Oxygen: oxygen that is dissolved in the blood plasma
Hemoglobin: a protein in red blood cells that binds to oxygen and carries it to the cells.
Myoglobin: a protein in muscle cells that stores oxygen for cellular respiration, one polypeptide chain which holds oxygen at low PO2
STRUCTURE OF HAEMOGLOBIN
Quaternary structure protein
Made of 4 polypeptide chains (4 globular subunits) associated with each other.
The four globin subunits are held together by disulphide bonds and arranged so that their hydrophobic R groups are facing inwards (helping preserve the three-dimensional spherical shape) and the hydrophilic R groups are facing outwards (helping maintain its solubility).
Each polypeptide chain contains a Haem group containing an iron ion (Fe2+) which combines with oxygen.
AFFINITY FOR OXYGEN
AFFINITY FOR OXYGEN is the tendency a molecule has to bind with oxygen.
Haemoglobin affinity for oxygen varies in the conditions it is in.
AFFINITY FOR PO2
PO2 is the partial pressure of oxygen.
The greater the concentration of dissolved oxygen in cells, the higher the partial pressure.
As PO2 increases, haemoglobin’s affinity for oxygen also increases:
Oxygen loads onto haemoglobin to form oxyhaemoglobin where there’s a high PO2.
Oxyhaemoglobin unloads its oxygen where there’s a lower PO2.
Loading of oxygen
ASSOCIATION/ LOADING IN THE LUNGS
High oxygen concentration so high PO2 as oxygen enters blood capillaries at the alveoli in the lungs.
High affinity for oxygen to bind.
Oxygen molecule binds/ loads to the iron in the haem group forming OXYHAEMOGLOBIN.
This slightly changes the conformation of the haemoglobin and makes binding sites available so more O2 binds easier.
It can carry 4 oxygen molecules (8 oxygen atoms)
unloading of oxygen
DISSOCIATION/ UNLOADING IN THE TISSUES
Low oxygen concentration so low PO2 as when cells respire, they use up oxygen which lowers the PO2
Low affinity for oxygen so it unloads.
Also, concentration of CO2 is high, increasing the rate of unloading (Bohr effect)
Oxygen is released from the haemoglobin molecule into respiring tissues.
SUMMARY OF AFFINITY OF HAEMOGLOBIN FOR O2
REGION IN BODY | O2 CONC (PO2) | CO2 CONC (PCO2) | AFFINITY FOR O2 | RESULT |
Gas exchange surface (lungs) | High | Low | High | Oxygen associates |
Respiring tissues | Low | High | Low | Oxygen dissociates |
OXYHAEMOGLOBIN DISSOCIATION CURVE: COOPERATIVE BINDING
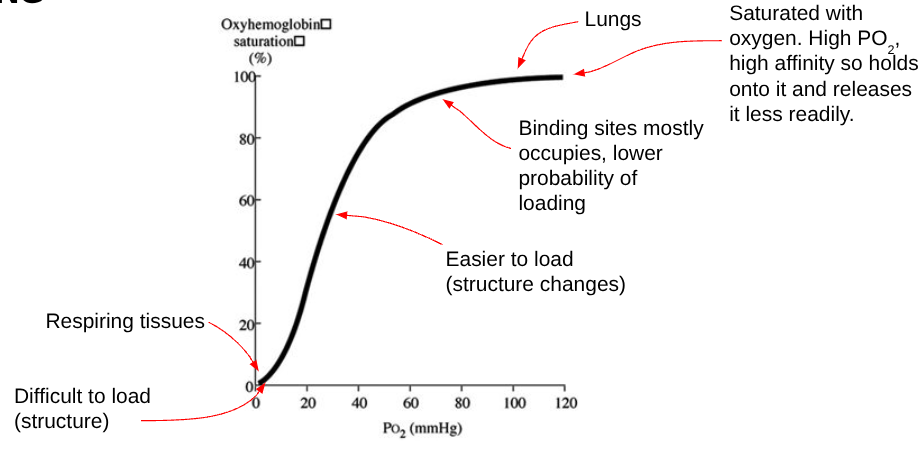
EXPLAINING THE CURVE (OXYHAEMOGLOBIN DISSOCIATION CURVE: COOPERATIVE BINDING)
The shape of the haemoglobin molecule makes it difficult for the first oxygen molecule to bind because the four polypeptide subunits are closely united. Therefore, at low pO₂ (respiring tissues), haemoglobin has a low affinity for oxygen, so little oxygen binds and the gradient of the curve is shallow.
Binding of the first oxygen molecule changes the quaternary structure of haemoglobin, causing it to change shape. This makes it easier for the remaining subunits to bind oxygen, so subsequent oxygen molecules bind more easily.
As a result, a smaller increase in pO₂ is needed to bind the second and third oxygen molecules, causing the gradient of the curve to steepen. This is known as positive cooperativity.
Once most binding sites are occupied, it becomes less likely that oxygen will bind, so the gradient decreases and the curve plateaus. At high pO₂ (alveoli), haemoglobin has a high affinity for oxygen and becomes fully saturated, allowing efficient oxygen loading.
THE BOHR EFFECT - EFFECT OF CARBON DIOXIDE
Haemoglobin has a reduced affinity for oxygen in the presence of carbon dioxide. The greater the concentration of carbon dioxide, the more readily the haemoglobin releases/dissociates with its oxygen.
High carbon dioxide concentration causes the oxyhaemoglobin curve to shift right. For the same PO2, the saturation of oxygen is much lower as the affinity decreases so more oxygen unloads in higher PCO2.
This is because in water (in blood) carbon dioxide forms carbonic acid and this acidic carbon dioxide slightly changes the tertiary structure of the haemoglobin. This happens at respiring tissues as respiration produces carbon dioxide (carbonic acid). In alveoli there is a lower partial pressure of carbon dioxide as it diffuses out of the blood so there is a higher affinity for oxygen.
EFFECT OF CARBON DIOXIDE ON HAEMOGLOBIN
In tissues, carbon dioxide is produced by respiring cells.
Carbon dioxide forms carbonic acid in solution, so pH of the blood within tissues is lowered.
The lower pH changes the conformation/shape/ tertiary structure of the haemoglobin into one with a lower affinity for oxygen.
Haemoglobin releases its oxygen to the respiring tissues.
At the gas exchange surface (alveoli) carbon dioxide is constantly being removed.
The pH is raised due to the low level of carbon dioxide at the exchange surface.
This higher pH changes the shape of haemoglobin into one that enables it to load oxygen readily to transport it to the respiring tissues.
The haemoglobin has a high affinity for oxygen when it is transporting it to the tissues so it is not released before the tissues.
DIFFERENT HAEMOGLOBINS
Structure of haemoglobin is coded for by DNA.
If there is a mutation that changes the shape of haemoglobin which is beneficial for the animal, by natural selection this allele will increase in the population.
There are also mutations which can cause harm i.e sickle cell anaemia.
A genetic disorder that causes abnormal haemoglobin, resulting in some red blood cells assuming an abnormal sickle shape and cannot carry as much oxygen
DISSOCIATION CURVES
The further to the left the curve, the greater the affinity of haemoglobin for oxygen (so it readily loads oxygen but unloads it less easily).
The further to the right the curve, the lower the affinity of haemoglobin for oxygen (so it loads oxygen less readily but unloads easily).
factors effecting the dissociation curves
CONC OF OXYGEN: the higher the concentration of oxygen, the more oxygen that can be transported by hemoglobin.
ALTITUDE: at higher altitudes, the air pressure and oxygen concentration decreases, making it harder for hemoglobin to transport oxygen.
TEMPERATURE: higher temperatures increase the solubility of oxygen in the blood, making it easier for hemoglobin to transport oxygen.
ACIDITY: changes in the acidity of the blood can affect the ability of hemoglobin to bind to oxygen.
FETAL HAEMOGLOBIN
The haemoglobin of a developing foetus has a higher affinity for oxygen than adult haemoglobin.
This is vital as it allows a foetus to obtain/attract oxygen from its mother's blood at the placenta. Fetus can’t inhale/exhale, mother haemoglobin is the only oxygen source.
Fetal haemoglobin can bind to oxygen at low PO2.
At this low PO2 the mother's haemoglobin is dissociating with oxygen.
On a dissociation curve graph, the curve for foetal heamoglobin shifts to the left of that for adult haemoglobin
This means that at any given PO2, fetal haemoglobin has a higher percentage saturation than adult haemoglobin
LOWER PO2 (HIGH ALTITUDE/UNDERGROUND)
The PO2 is lower at higher altitudes and underground.
Organisms that live in environments with a low concentration of oxygen have haemoglobin with a higher affinity for oxygen than human haemoglobin.
This is beneficial as it allows them to obtain/attract a sufficient level of oxygen saturation in their blood when the PO2 in the air is low.
The dissociation curve of their haemoglobin is to the left of ours, higher affinity, higher saturation at any PO2.
HIGH ACTIVITY LEVELS
Organisms that are very active and have a high oxygen demand have haemoglobin with a lower affinity for oxygen than human haemoglobin.
This is because they need their haemoglobin to easily unload/ dissociate oxygen, so that it’s available for them to use.
The dissociation curve of their haemoglobin is to the right of the human one.
MYOGLOBIN
Myoglobin is a single polypeptide chain in a tertiary structure, it has only one oxygen binding site.
This means it is fully saturated after one oxygen molecule has bound to it.
It also does not do cooperative bonding.
Myoglobin’s affinity for oxygen is higher than the haemoglobin, specifically at lower levels.
This is due to the fact that myoglobin has a simpler job than haemoglobin which is to store and release oxygen to the muscles whereas, haemoglobin is also responsible for carrying and releasing the oxygen at the right places.
DISSOCIATION CURVE SUMMARY
CONDITION | SHIFTS | AFFINITY | LOAD/UNLOAD | WHY? |
Carbon dioxide concentration Bohr Effect. | Right | Lower | Unload | Carbon dioxide forms carbonic acid and this acidic carbon dioxide slightly changes the tertiary structure of the haemoglobin so it more readily dissociates at respiring tissues. |
Fetal | Left | Higher | Load | To obtain/attract oxygen from its mother's blood at the placenta. Fetus can’t inhale/exhale, mother haemoglobin is the only oxygen source. |
Low oxygen environments | Left | Higher | Load | To obtain/attract a sufficient level of oxygen saturation in their blood when the PO2 in the air is low. |
High SA:V (small animal) and fast metabolism. | Right | Lower | Unload | Small animals have a higher surface area to volume ratio, meaning they lose heat quickly. They need a high metabolic rate to help them keep warm. This means they have a high oxygen demand. They need their haemoglobin to easily unload oxygen. |
High activity | Right | Lower | Unload | High O2 demand, need oxygen to easily unload to use. |
Myoglobin | Left | Higher | Load | Only one oxygen to bind until full saturation. |