Biochemistry Chapter 2
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Name the 5 hierarchical levels of molecules important in biological systems
Inorganic precursors (CO2, H2O, NH3, N2)
Metabolites (Pyruvate, Citrate, Succinate)
Building blocks (Amino acids, Nucleotides)
Macromolecules (Proteins, Nucleic acids)
Supramolecular Complexes (Ribosomes, Cytoskeleton)
Properties of molecules is based on what?
Composition
Covalent structure
Bonding and molecular geometry
What space will electrons occupy?
Space with minimum repulsion. As far away from each other as possible.
Chemical bonds allow what?
The sharing of electrons between the nuclei of the two bonded atoms. Can be shared equally or unequally.
What does degree of electron sharing determine about a bond?
Strength of bond
Energy associated with the bond
Define electronegativity
The ability of an atom within a molecule to draw electrons towards itself. When EN is equal between atoms electron is equally shared.
Explain the difference between polar and non-polar covalent bonds.
Non-polar, electrons are shared equally. H2.
Polar, electrons reside closer to nucleus with greater ability to attract them. HCl.
Define hydrogen bond in relation to water.
Electrostatic attraction between the oxygen atom of one water molecule and the hydrogen of another.
What properties of water are higher due to hydrogen bonding?
Melting point
Boiling point
Heat of vaporization
Surface tension
Are H bonds shorter or longer than covalent?
Longer
What can be said about the strength and lifetime of H-bonds?
BDE = ~23kJ/mol in H2O
Polar bonds
10% covalent, 90% electrostatic
Strong dipole-dipole or charge-dipole interactions
Strongest when bonded molecules allow for linear bonding patterns
Bond lifetime in liquid is 1-20 picoseconds
When one H bond breaks, another forms
Why are H bonds biologically important?
Sources of unique properties of water
Structure and function of proteins
Structure and function of DNA
Structure and function of polysaccharides
Binding of substrates to enzymes
Binding of hormones to receptors
Matching of mRNA and tRNA
H’s bonded to what atoms can participate in hydrogen bonding?
F, O, N (Fluorine, Oxygen, Nitrogen)
Phase changes from solid to liquid and liquid to gas for water are spontaneous but endothermic. What must increase to drive these changes?
Entropy (S)
Up to how many H bonds can water form in its liquid state?
3
Up to how many H bonds can water form in its solid state?
4
What is the result of water being able to form more H bonds in its solid state?
A lower density than liquid water. Ice floats.
Define hydrophilic
Compounds that dissolve easily in water; generally charged or polar compounds
Define hydrophobic
Compounds that do not dissolve easily in water; nonpolar molecules such as lipids and waxes
Define amphipathic
A compound that contains regions that are polar (or charged) and regions that are nonpolar
Water is a good solvent for what types of substances?
Charge and polar
Amino acids
Peptides
Small alcohols
Carbohydrates
Water is a poor solvent for what types of substances?
Nonpolar
Nonpolar gases
Aromatic moieties
Aliphatic chains
How is force of ionic interactions in solution calculated?
Q = Magnitude of charges
r = distance between charged group
E = dielectric constant of the solvent (Water at 25 degree Celsius = 78.5)
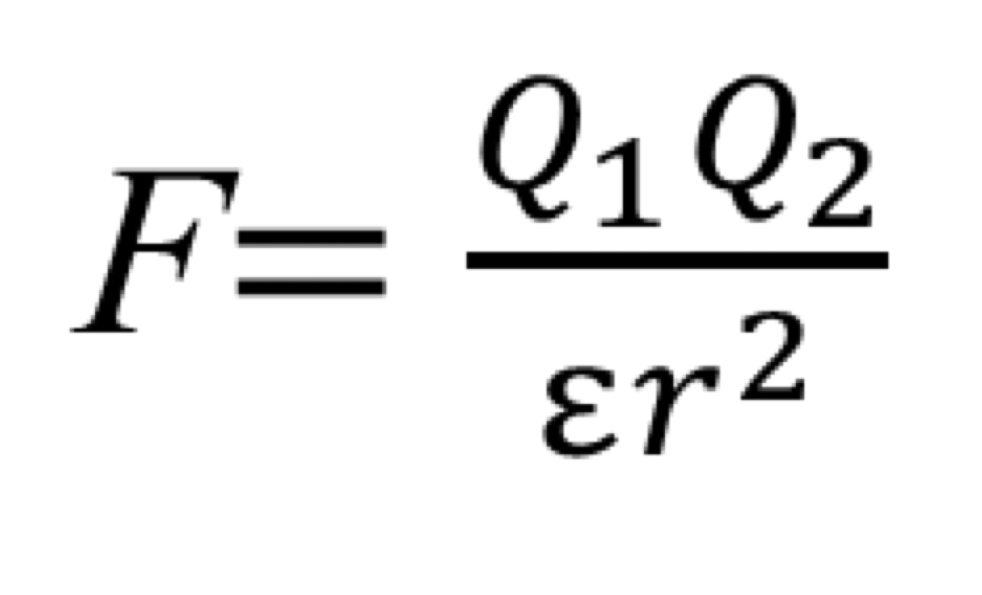
Over what distance do ionic attractions or repulsions operate?
10-40 nm
How does a high dielectric constant affect attraction between oppositely charged ion in a salt crystal?
Reduces it.
As a crystal lattice is dissolved what happens to entropy?
It increases
How do strong electrostatic interactions between the solvated ions and water molecules affect the energy of the system?
Lowers it.
How does water dissolve most crystalline salts?
By hydrating their component ions. Ionic charges are partially neutralized and electrostatic attractions needed for lattice formation are weakened.
What types of non-covalent interactions exist?
Ionic interactions
Dipole interactions
van der Waals interactions
Hydrophobic effects
What two components make up van der Waals interactions and what do they depend on?
Attractive force (London dispersion) depends on polarizability
Repulsive force (Steric repulsion) depends on size of atoms
Explain the importance of van der Waals interactions
Universal occur between any two atoms near each other
Weak individually; easily broken and reversible
Determines steric complementarity
Stabilizes biological macromolecules (base stacking in DNA)
Facilitates binding of polarizable ligands
What is the hydrophobic effect and what biological processes is it the driving force for?
Refers to the association or interaction of nonpolar molecules or components of molecules in the aqueous solution.
Main factor behind:
Protein folding
Protein-protein association
Formation of lipid micelles
Binding of steroid hormones to their receptors
Water surrounding nonpolar solutes has lower or higher entropy?
Lower
Micelle formation is thermodynamically favored why?
The ordered shell of H2O molecules is minimized and entropy is increased