Properties of Solutions: Key Concepts for ATI TEAS 7
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What is a solution?
A uniform mixture of two or more substances
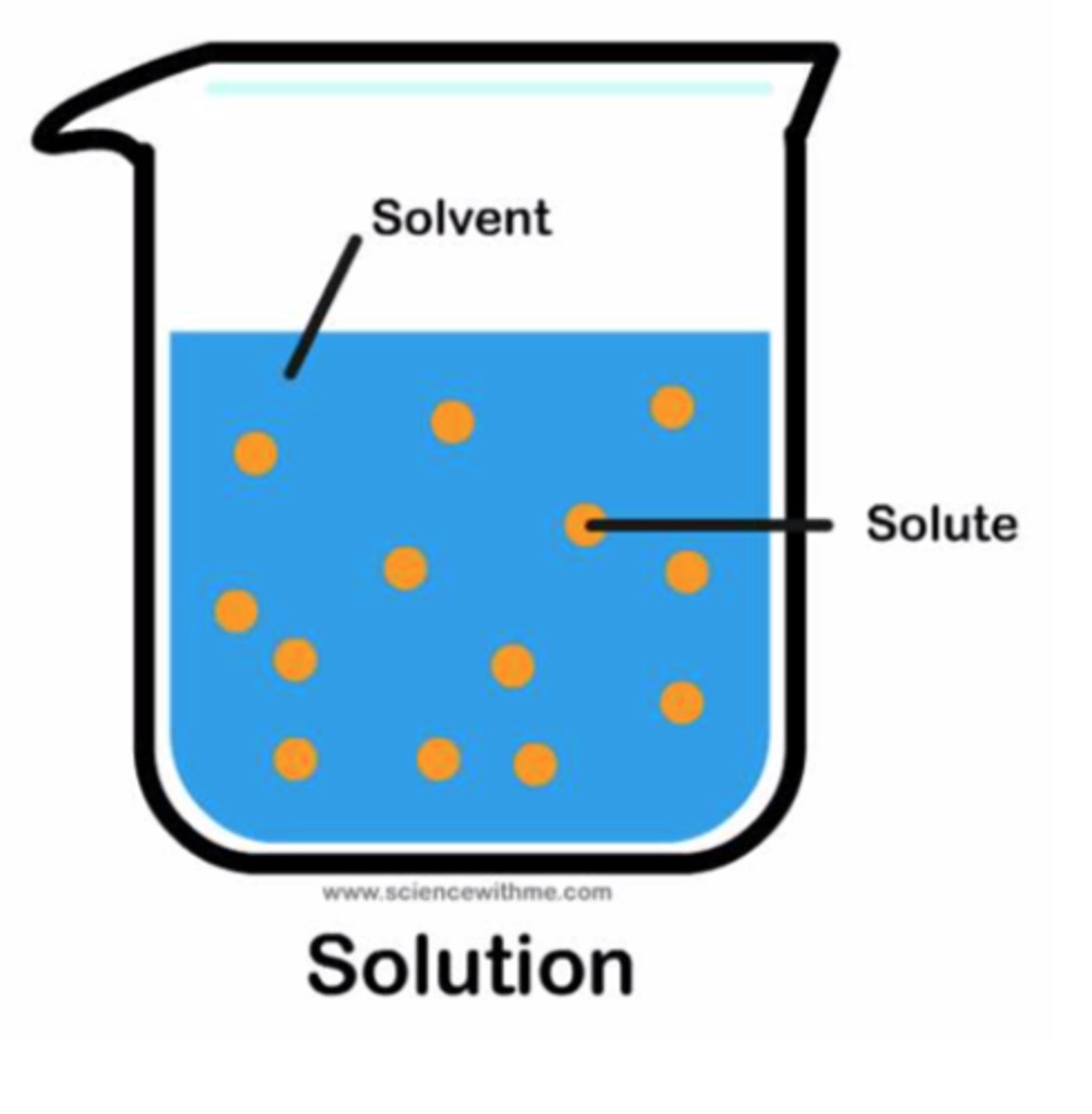
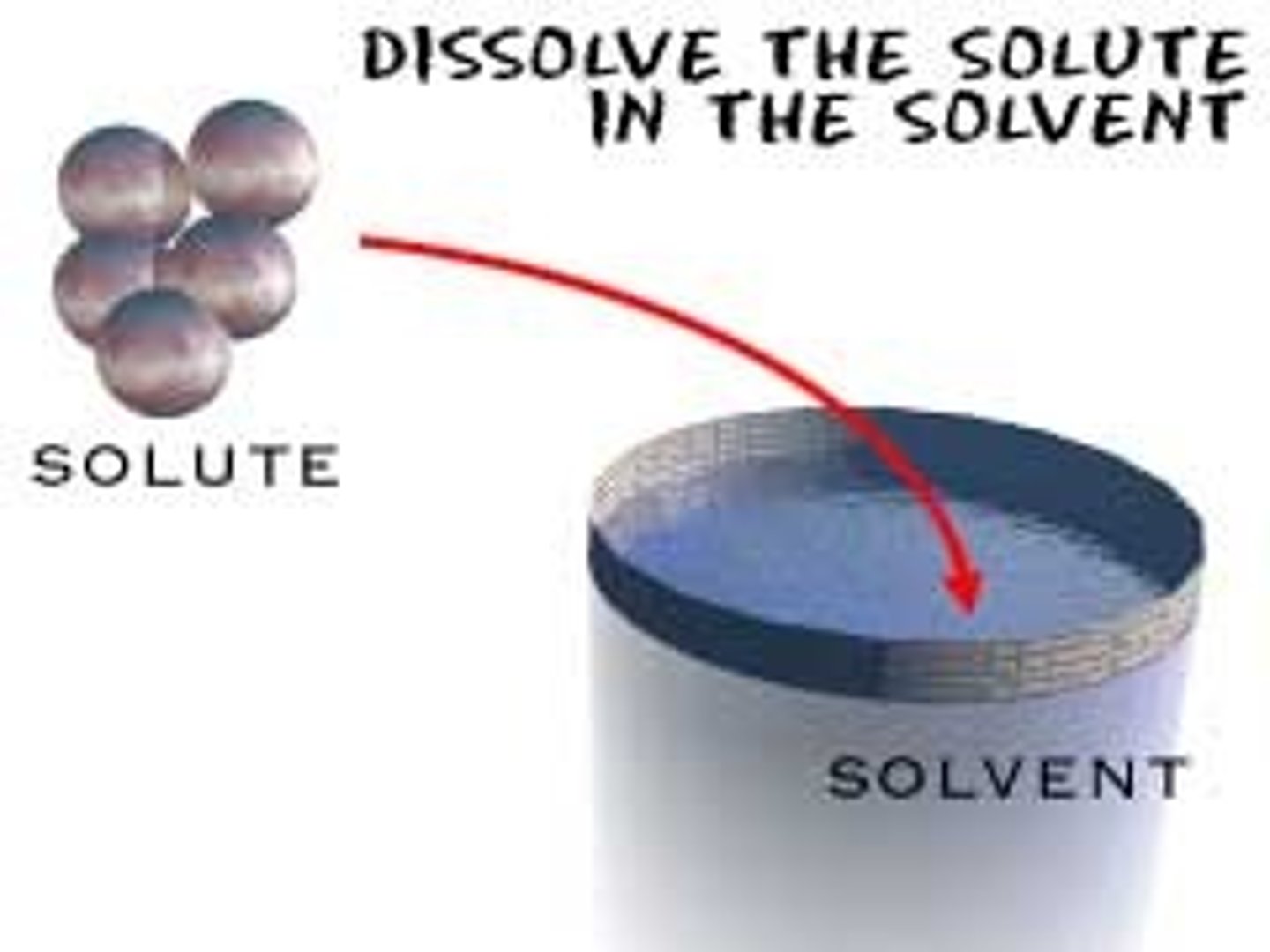
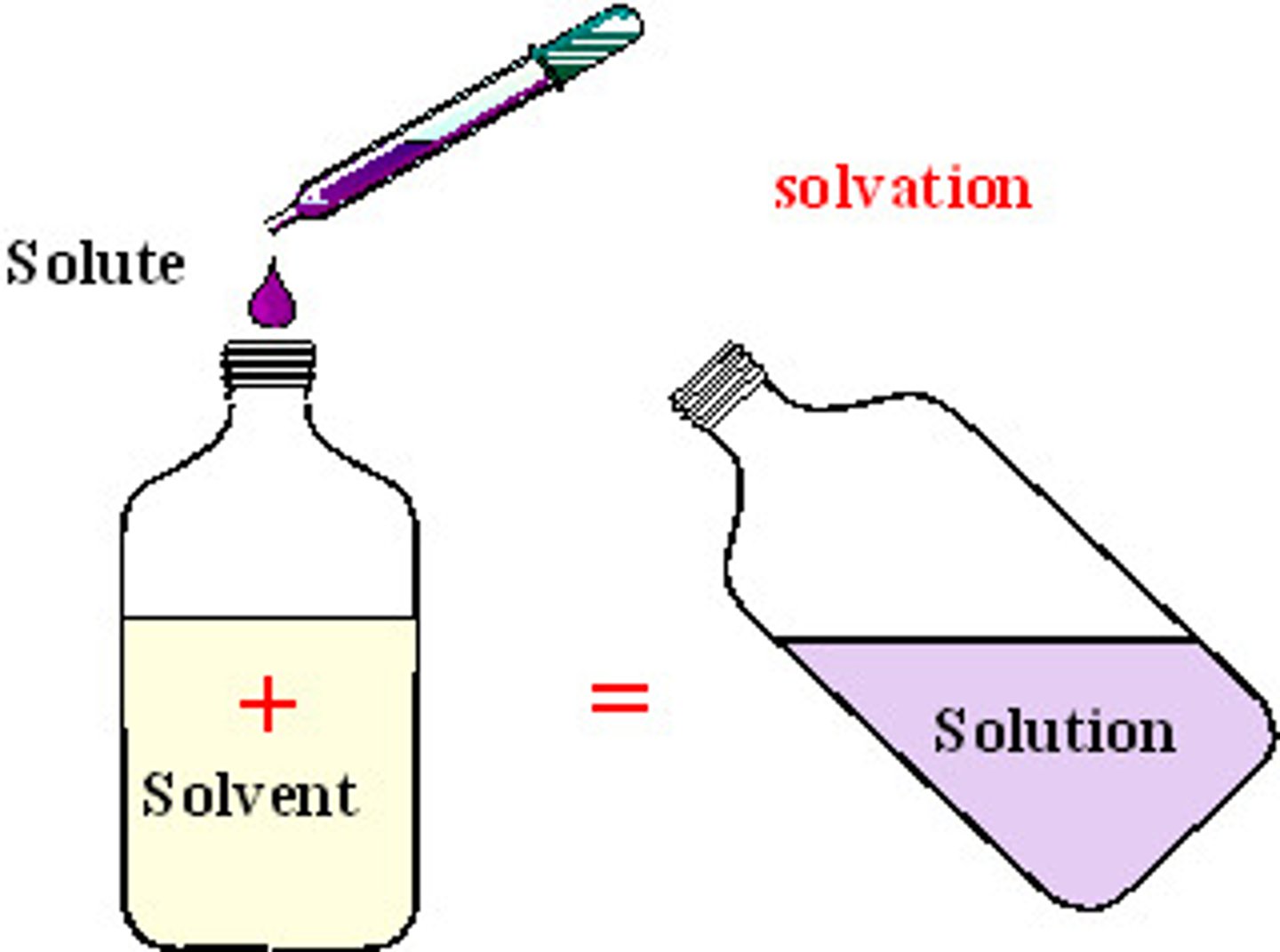
What is a solute?
Substance being dissolved
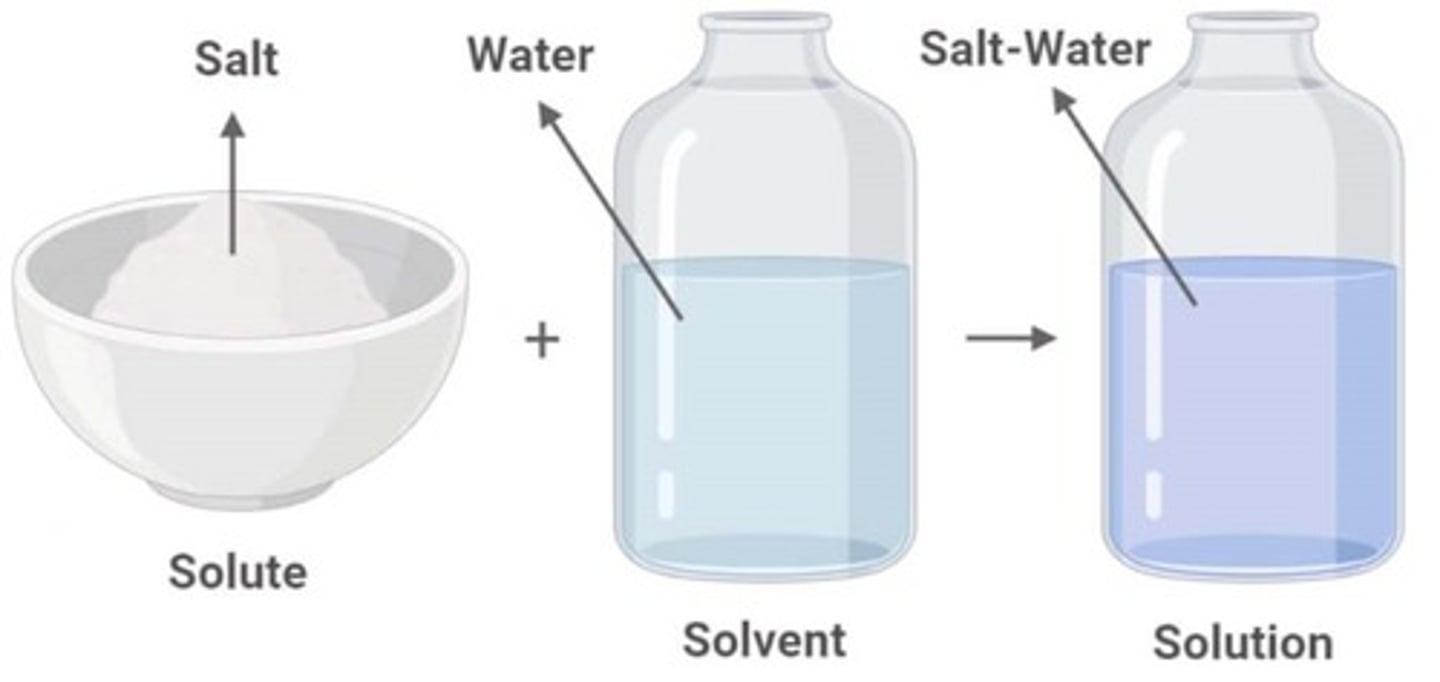
What is a solvent?
Substance that dissolves the solute
Which is present in greater amount in a solution?
Solvent
What is molarity (M)?
Concentration of a solution measured in moles per liter
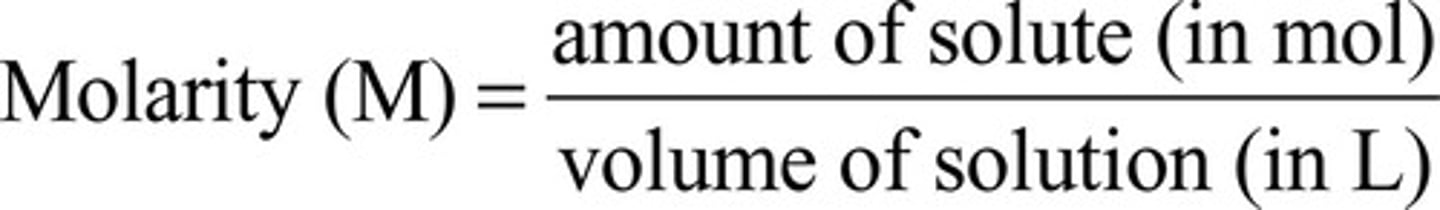
What does high molarity mean?
More solute in solution

What does low molarity mean?
Less solute in solution
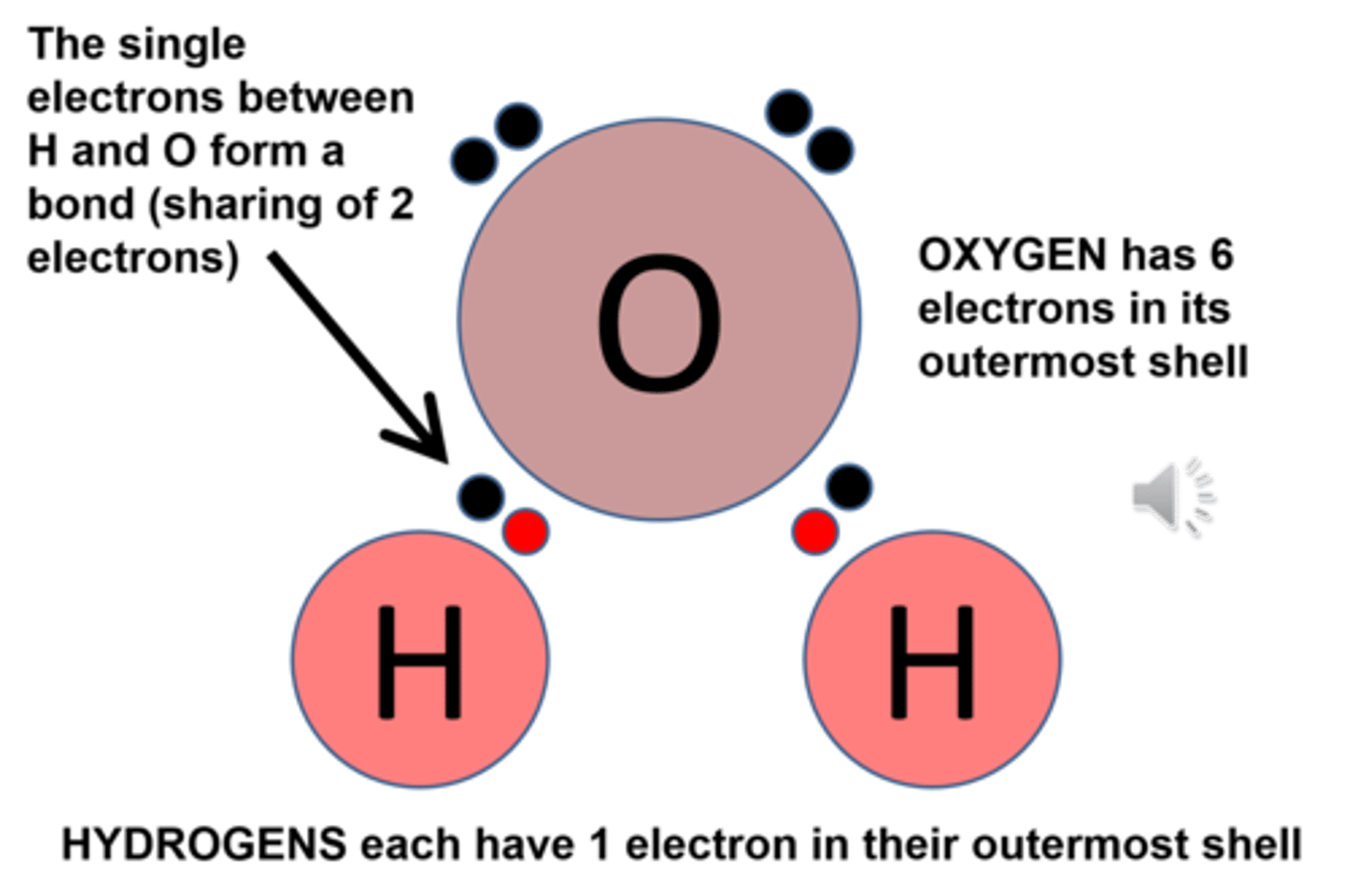
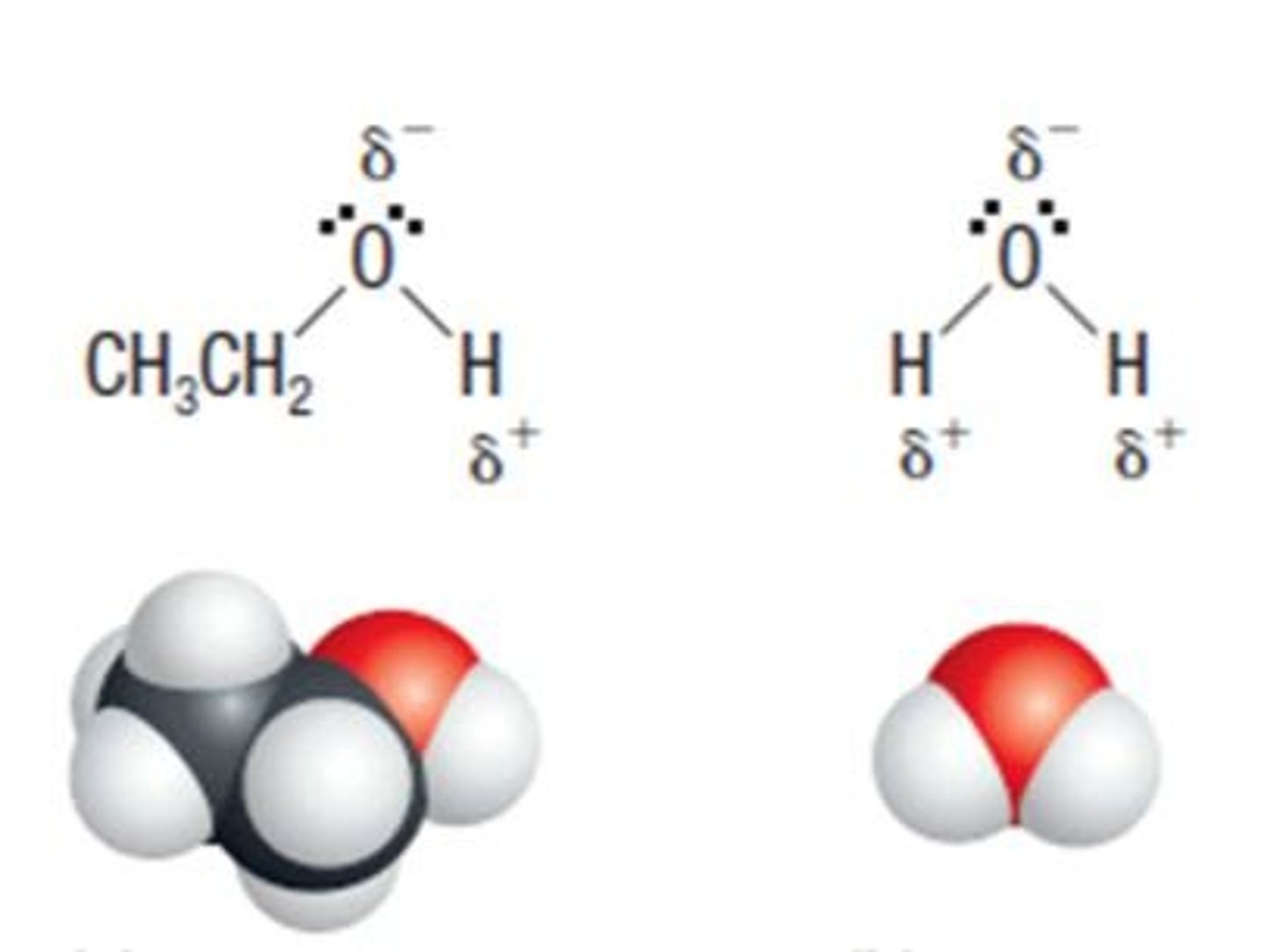
Why is water a good solvent?
Because it is polar
What is polarity?
Uneven distribution of electrical charge
What is hydrogen bonding?
Attraction between water molecules due to polarity
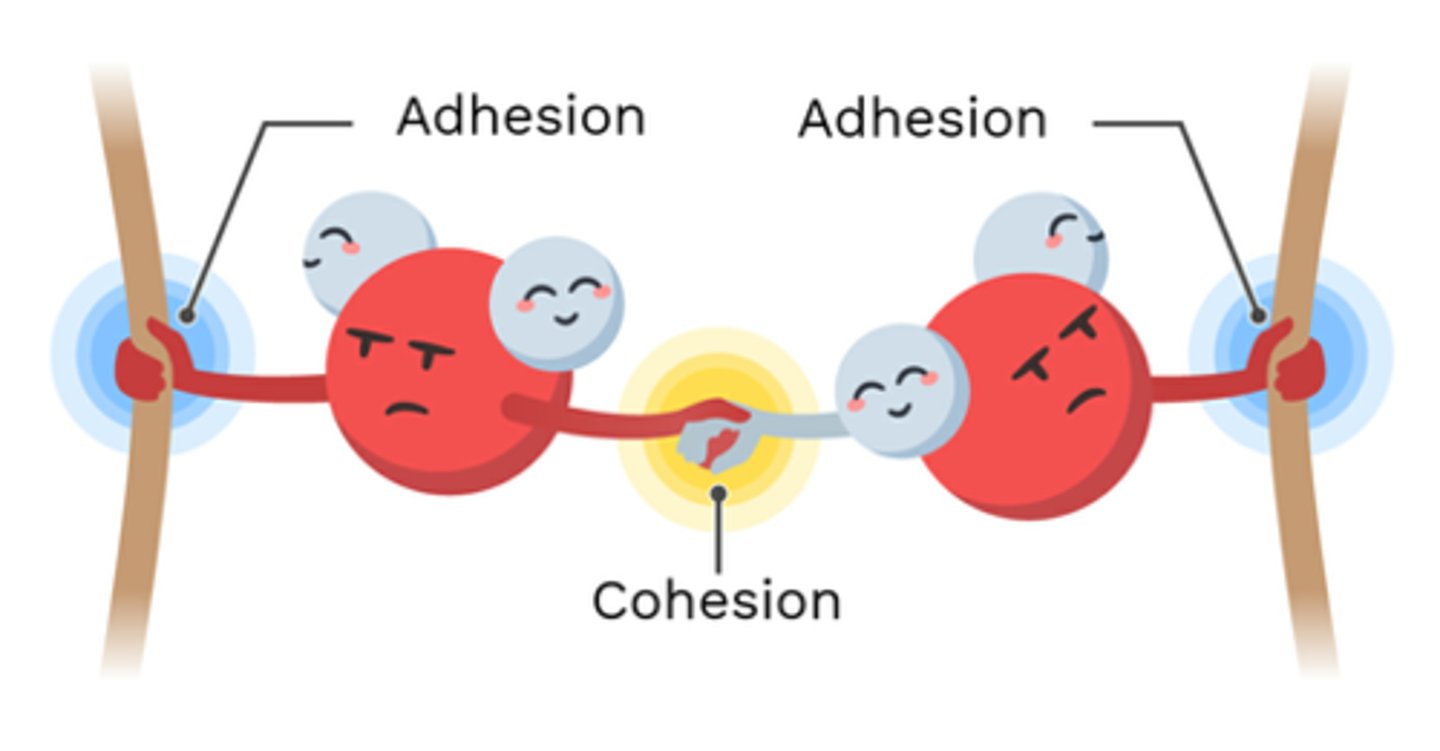
What is cohesion?
Water molecules sticking to each other
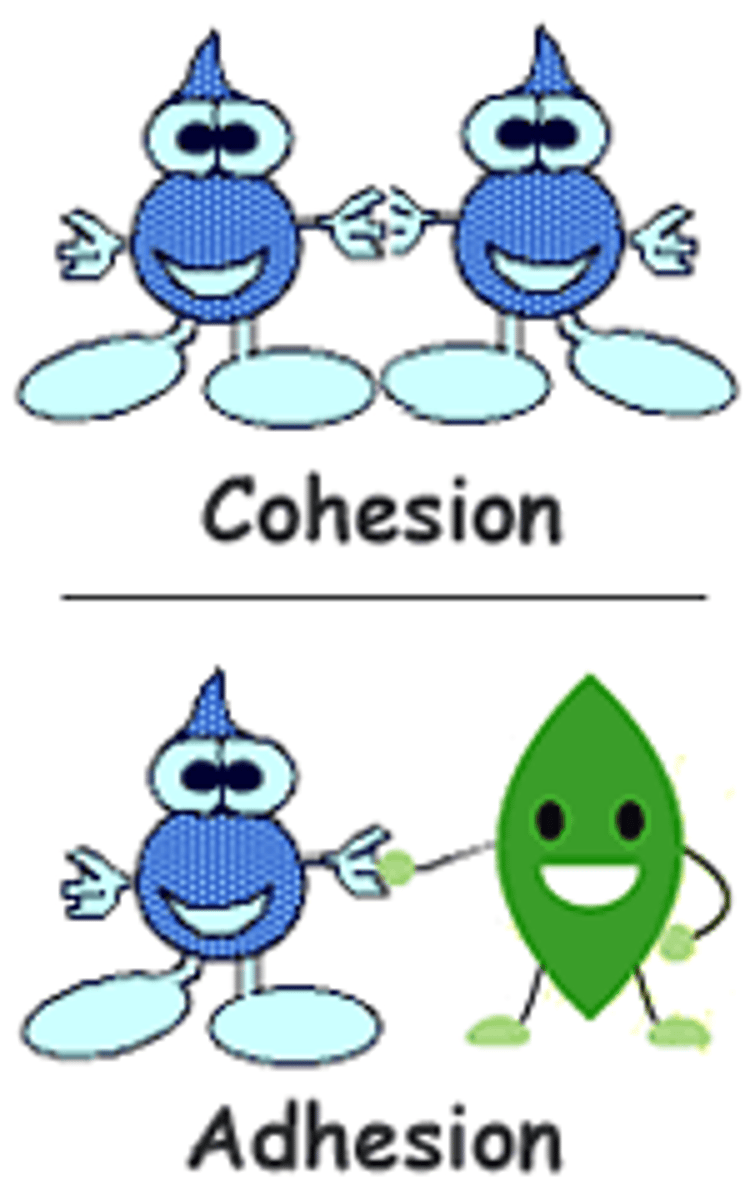
What is adhesion?
Water molecules sticking to other substances
What types of substances dissolve well in water?
Polar substances
What types of substances do not dissolve well in water?
Nonpolar substances
Give an example of a polar substance.
Sugar or salt
Give an example of a nonpolar substance.
Oil or fat
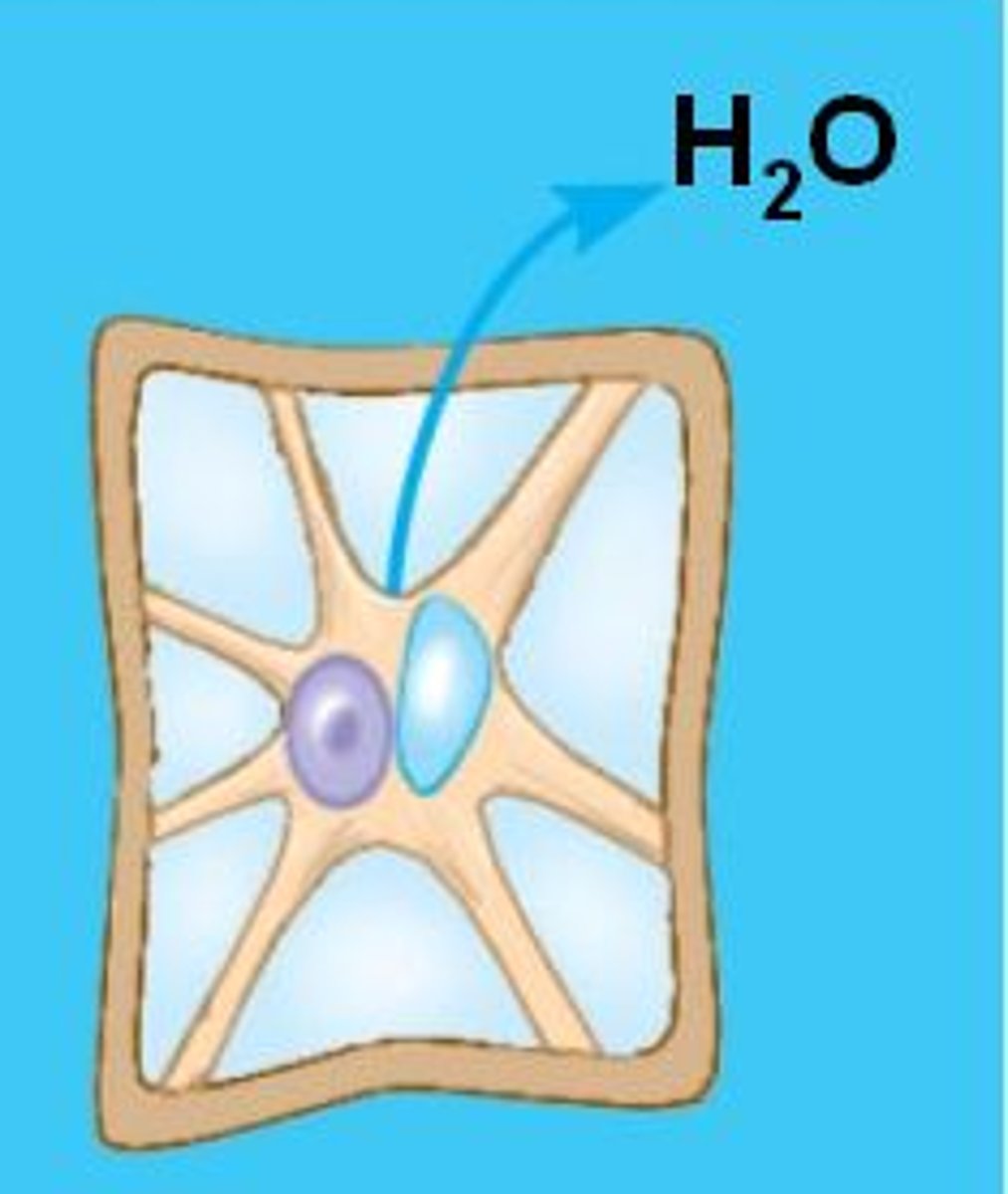
What is osmosis?
Movement of water across a selectively permeable membrane
From where to where does water move during osmosis?
From low solute concentration to high solute concentration
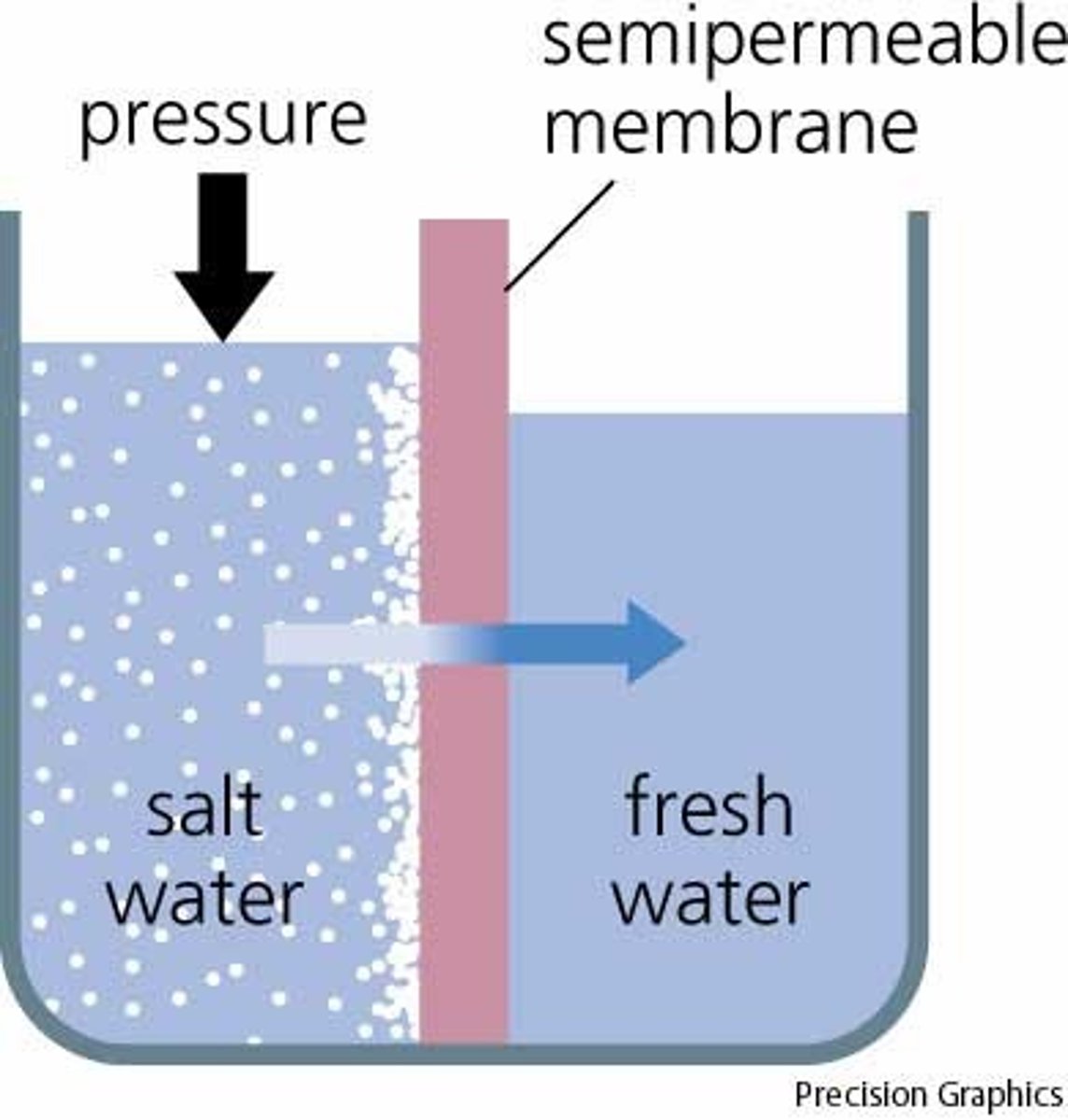
What is a selectively permeable membrane?
Allows some substances to pass but not others
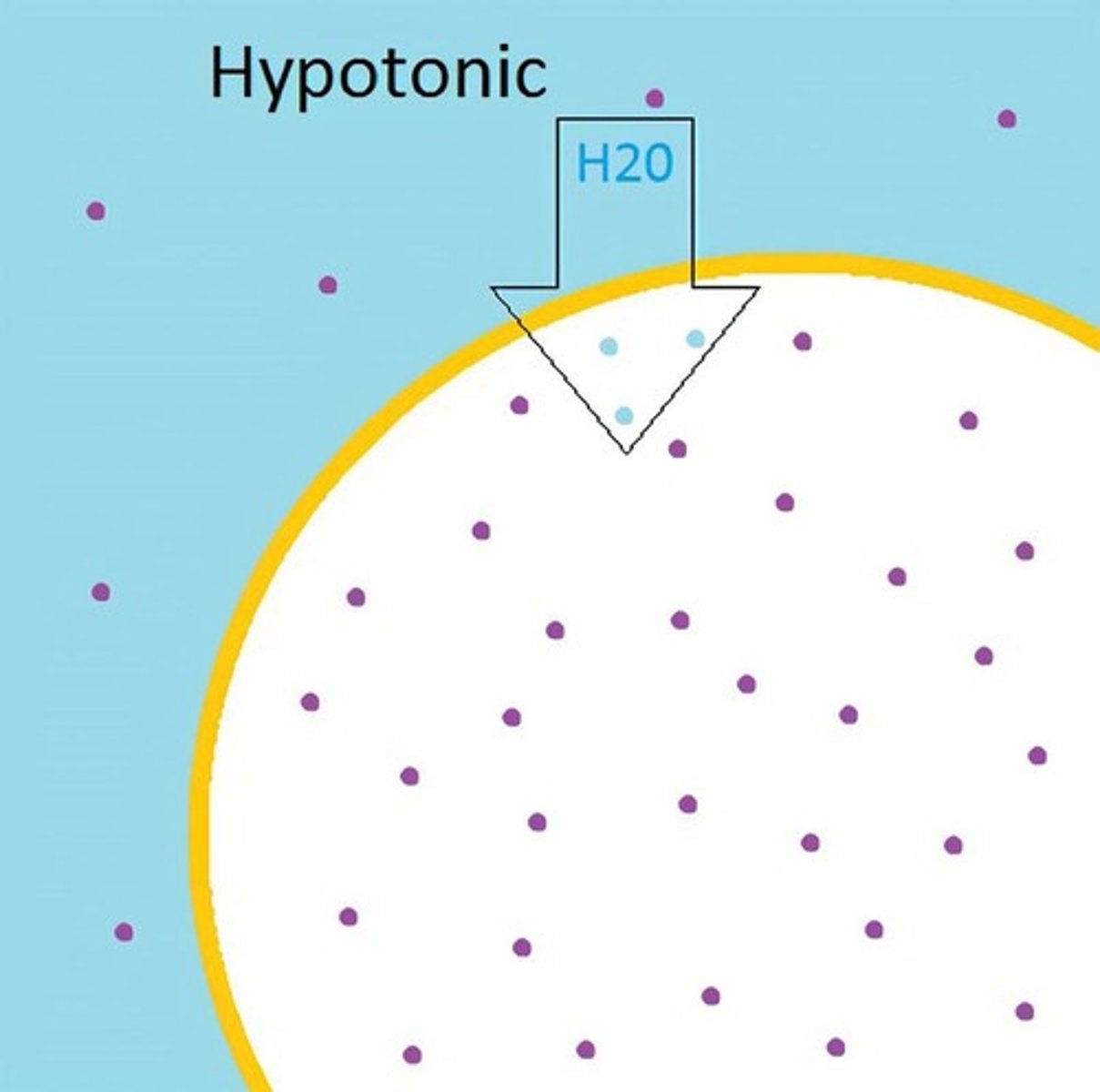
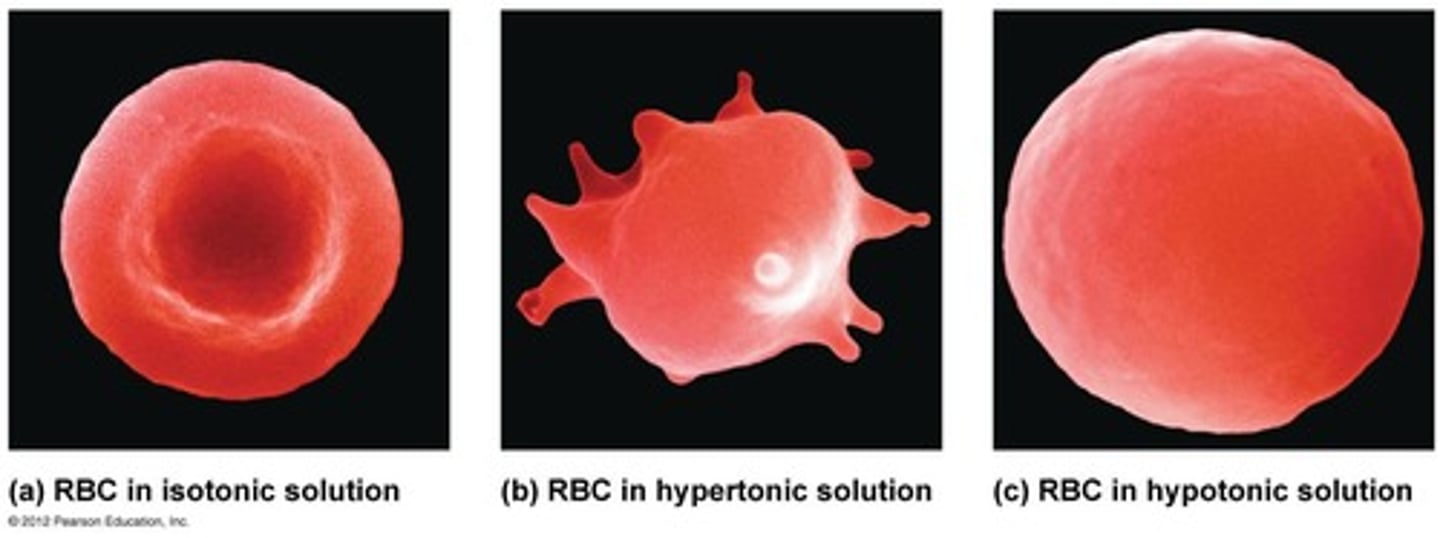
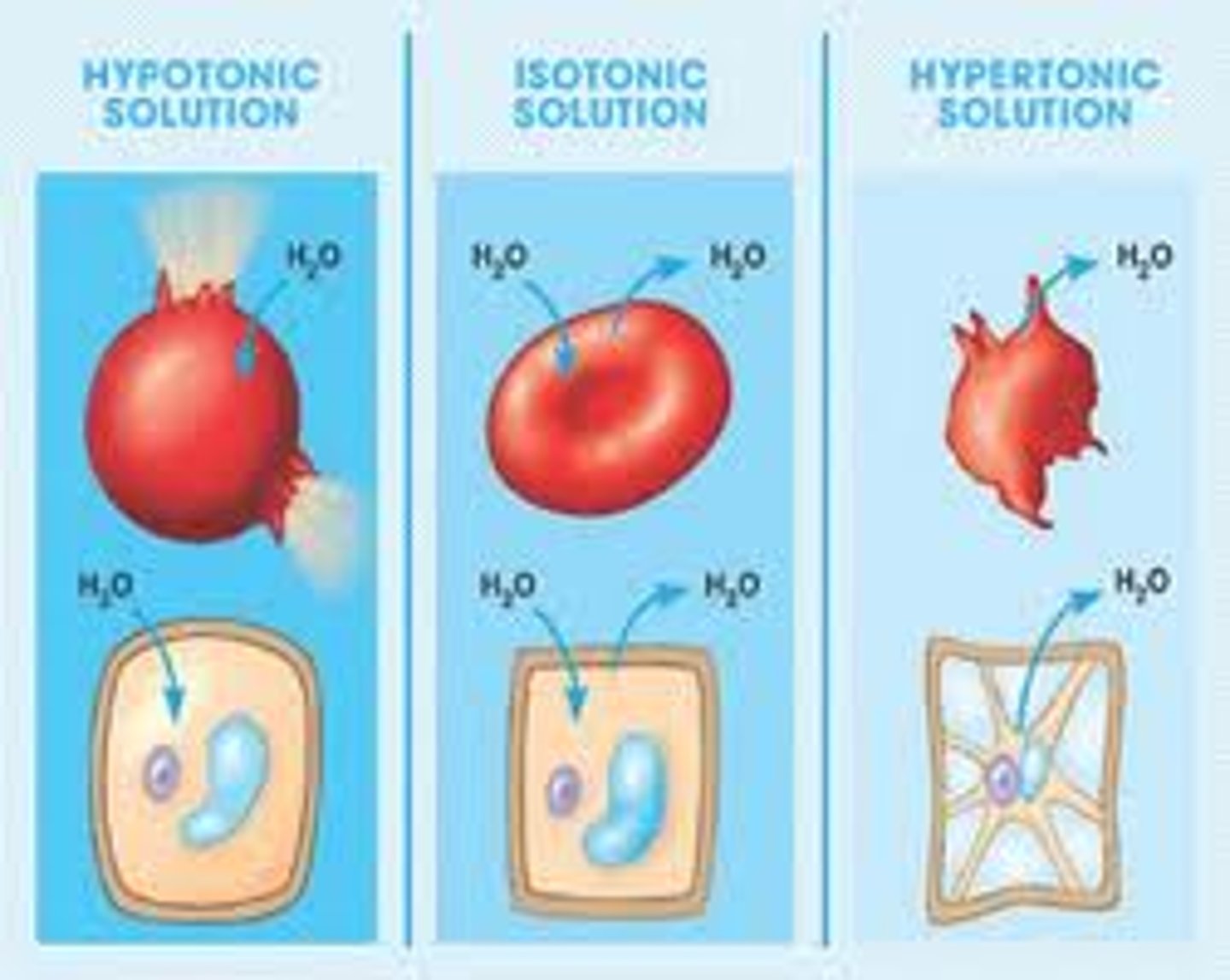
What happens in a hypotonic solution?
Causes cell to swell
What happens in an isotonic solution?
Causes no net movement of water
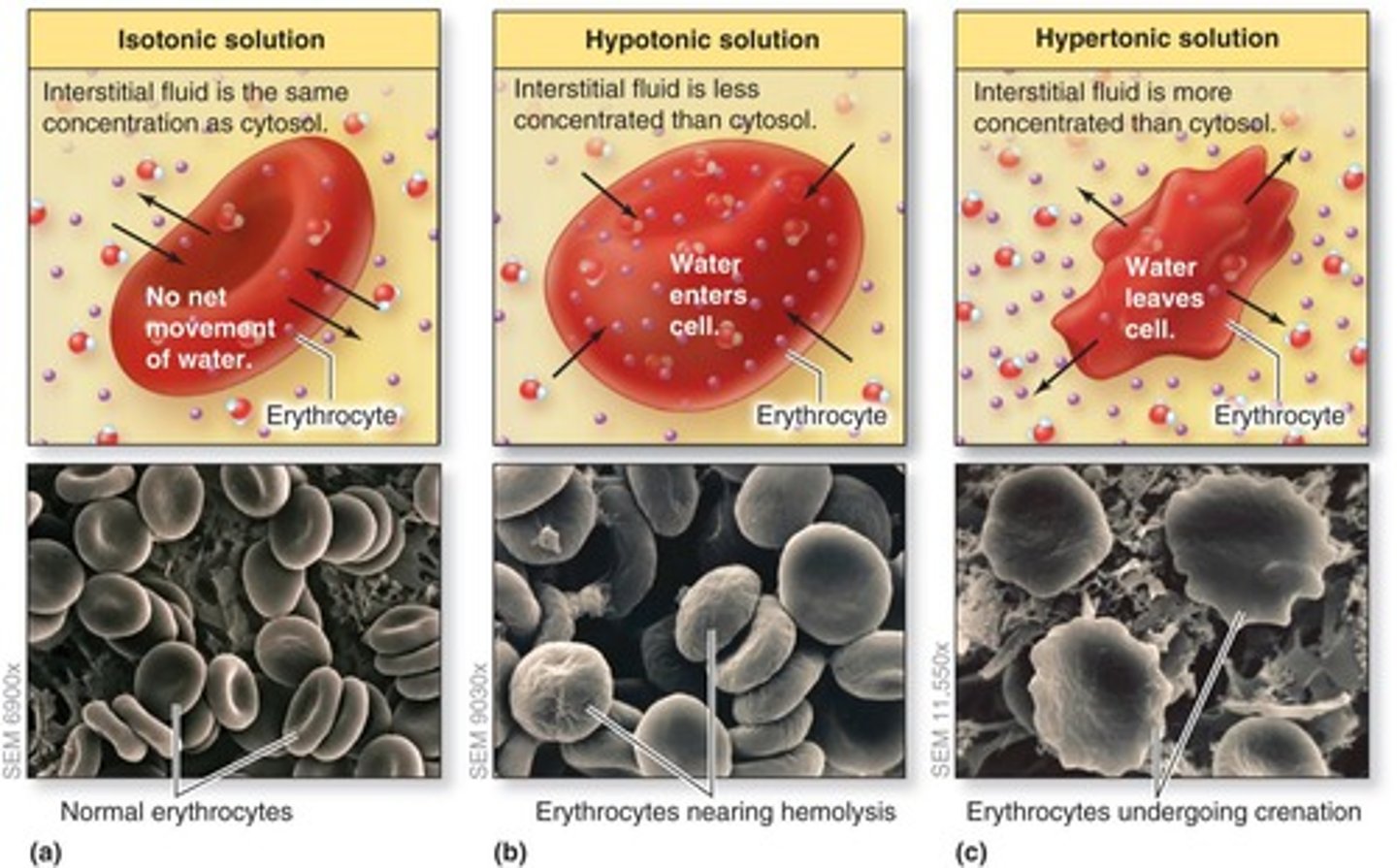
What happens in a hypertonic solution?
Causes cell to shrink
What does 'like dissolves like' mean?
Polar dissolves polar, nonpolar dissolves nonpolar
What moves during osmosis?
Water only
What is the difference between diffusion and osmosis?
Diffusion: solutes move; Osmosis: water moves
Still learning (13)
You've started learning these terms. Keep it up!