Unit 4 - Chemical Reactions
1/40
Earn XP
Description and Tags
Covers the ENTIRE Unit 4 of AP Chemistry (Units 4.1-4.9)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What is a physical change?
A change in properties but not a change in composition.
How do you typically know if something went through a physical change?
Changes in phase of a substance, formation/separation of mixtures.
In terms of IMFs, what do physical changes typically do to them?
It breaks IMFs.
What is a chemical change?
When substances are transformed into new substances, with different compositions and new properties.
How do you know if something was a chemical change?
If there is production of heat, light, a gas, precipitate, color change.
In terms of BONDS, what do chemical changes typically do?
They break bonds OR form new chemical bonds.
What kind of change is dissolving, why?
It can be both physical and chemical because ionic bonds ar ebroken and ion-dipole interactions are formed.
Key thing to know for changes is that Physical changes _____ while chemical changes ____.
typically break IMFs; break bonds OR form new bonds.
What are Net-Ionic Equations?
Both physical and chemical reactions that balanced equations can represent. They are helpful for single/double displacement.
How to Write Them:
Exclude spectator ions and ONLY SPLIT UP AQUEOUS THINGS into their respective ions! Everything else is written the same!
How do chemical reactions happen?
They happen whenever atoms are rearranged so that mass is conserved.
How would you represent a chemical reaction?
The diagrams must contain equal numbers of atoms of every element before and after the reaction.
What are the types of equations used to represent reactions?
Balanced molecular, complete ionic, and net ionic.
What are Complete Ionic Equations?
They show every substance in the reaction and split aqueous ionic compounds into their respective ions.
EX: Na+ (aq) + Cl- (aq) + Ag+ (aq) + NO3- (aq) → AgCl (s) + Na+ (aq) + NO3- (aq)
What are Molecular Equations?
They show every substance in the reaction.
What are Net Ionic Equations?
They take out spectator ions that do not participate in the chemical reaction to only focus on the ionics in the reaction.
EX: If chlorine stays aqueous on both sides, you don’t have to include it. (just know that they are not the exact same on both sides)
Why could you use particulate diagrams to represent reactions?
It can be used to represent the equations OR show how many excess particles would remain.
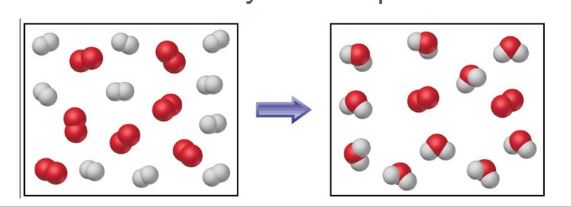
Why do we use stoichiometry?
It is used to show that atoms MUST be conserved during a chemical process. You can also use it to calculate product amonuts by using known reactant amounts to calculate other reactants/products.
List the conversion factors YOU MUST KNOW in terms of calculating things for stoich! (likely FRQ question)
Avogadro’s Number
Molar Mass (g/mol)
Molar volume of a Gas at STP (1 mol = 22.4L)
Molarity (mol/L)
What are titrations used for?
They are used to determine the concentration of an analyte.
What is an analyte?
Whatever you are measuring in a solution. (it is the thing that you are adding something to)
What is a titrant? Why do we add it?
The thing you are adding to the analyte. We add it because we know the concentration of it and can use it to calculate the concentration of the analyte through a reaction.
Solve this problem:
50mL of unknown HCl is slowly added from 100mL to 78mL of 0.1M NaOH. What is the concentration of HCl?
Step 1: Find the molarity of NaOH by subtracting 100 from 78 and turning into liters.
Step 2: Turn that into moles of NaOH (0.022L * 0.1 mol NaOH)
Step 3: Turn that into moles of HCl (0.0022m *1/1)
You need to write the reaction, then balance it via coefficients
Step 4: You now have moles of HCl so divide that by volume of HCl to get the concentration: 0.0022mol/0.05=0.044
REMEMBER, YOU MUST SHOW ALL OF YOUR WORK!
What are the three types of chemical reactions?
Oxidation-Reduction, Precipitation, and Acid-Base.
What is a Precipitation Chemical Reaction?
It involves mixing ions in aqueous solutions to produce an insoluble (solid) or barely soluble ionic compound. (aq —> s)
YOU MUST KNOW THIS. What salts are soluble in water?
All sodium, potassium, ammonium, and nitrate salts.
What is an acid-base reaction?
It involves the transfer of one or more protons between chemical species.
What is the Bronsted-Lowry Acid definition?
A substance that donates protons (H+) in a chemical reaction.
What is the Bronsted-Lowry Base definition?
A substance that receives protons (H+) in a chemical reaction.
Acids will (increase/decrease) the concentration of (H3O+/OH-) in a solution?
increase; H3O+
Bases will (increase/decrease) the concentration of (H3O+/OH-) in a solution?
decrease; OH-
What is an oxidation-reduction reaction?
It involves the transfer of one or more electrons between chemical species, as indicated in oxidation numbers.
How are electrons transferred in oxidation-reduction reactions?
They are transferred from the species that is oxidized to the species that is reduced.
What is combustion? When does it occur? In hydrocarbons, what are products?
Combusion is a chemical process in oxidation-reduction reactions when a species reacts with oxygen gas to form CO2 and H
It happens in oxidation-reduction reactions when a species reacts with oxygen gas. In hydrocarbons, CO2 and H2O are products of this.
What is an atom in an elements oxidation number?
0
What is a monotomic ions oxidation number?
Its charge
What is fluorines oxidation number?
-1 in its compounds
What is oxygens oxidation number?
-2 in its compounds (usually)
What is hydrogens oxidation number?
+1 in its covalent compounds
What are peroxides containing O2-2 oxidation number?
-1 in its compound
What happens if you have a compound in terms of oxidation numbers?
They must total to 0 for neutral compounds or the charge of a chaged compound/polyatomic ion.
In terms of half-reactions, they are used for huge reactions. You need to do practice on this because I am not including it in this flashcard set.
Okay!