arenes and aromaticity
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
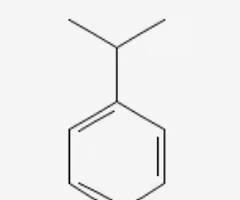
Cumene
benzene + isopropyl
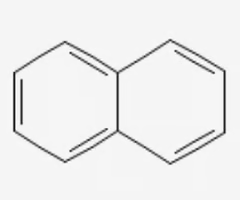
naphthalene
2 benzene rings
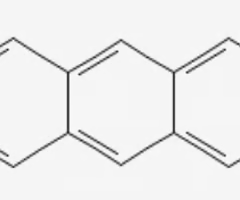
anthracene
3 benzene rings (straight)
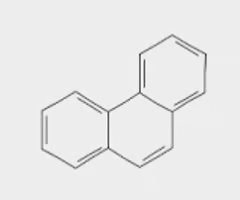
phenanthrene
3 benzene rings (off)
bond length of alkane
150
bond length of alkene
130
bond length of whole benzne
~140
structure of a benzene
electrons are not localized
circle in a ring stands for resonance
possesses aromacity
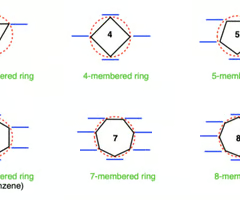
frost circle
wherever polygon touches circle, there is an energy level
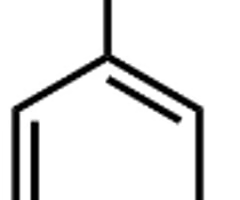
halobenzene (ex. bromo benzene)
benzene with a halogen substituent
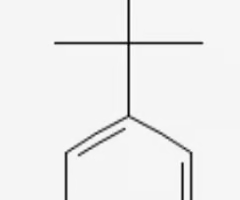
tertbutyl benzene
benzene connected to tertbutyl group
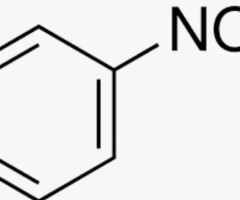
nitrobenzene
benzene connected to nitro group
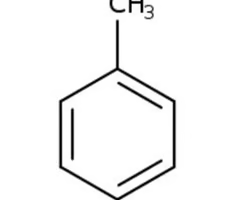
toulene
benzene connected to methyl group
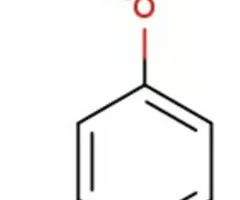
methoxcy benzene/anisole
ether with a benzene
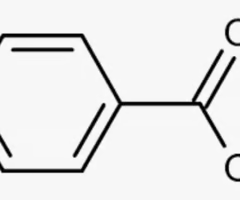
benzoic acid
benzene with carboxylic acid
benzaldehyde
benzene with aldehyde
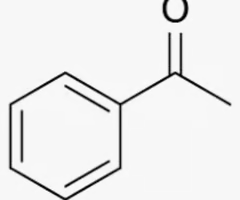
acetophenone
benzene with a ketone
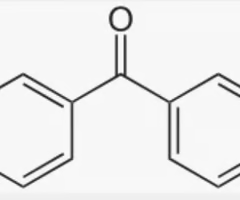
benzophenone
2 benzenes connected with ketone
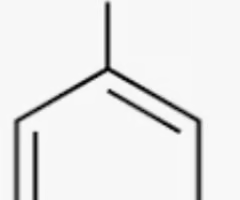
phenol
benzene with alcohol
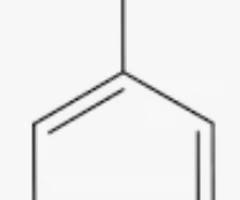
aniline
benzene with NH2
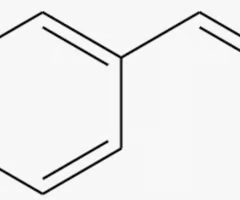
styrene
benzene with a alkene
naming
substituents in alphabetical order
lowest numbers possible
ortho
1,2
meta
1,3
para
1,4
physical properties of arenes
nonpolar
insoluble in water
less dense than water
carcinogenic
reactions occur at side chain or at ring
LUMO
lowest occupied molecular orbital
HOMO
highest occupied molecular orbital