Everything Food Technology
1/238
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
239 Terms
(FPE) Where does coffee crema come from?
Coffee crema forms from the emulsification of coffee oils and gases, primarily carbon dioxide, during the espresso extraction process. The pressure forces water through the coffee grounds, creating tiny bubbles that rise to the surface as a creamy, golden-brown foam.
(FPE) Where does tea cream come from?
A precipitate of polyphenols with divalent ions
(FPE) process of coffee roasting in detail
Initially low temperature to remove water
Then temp increases and beans expand and become porous
Coffee beans are heated in an environment that contains little oxygen.
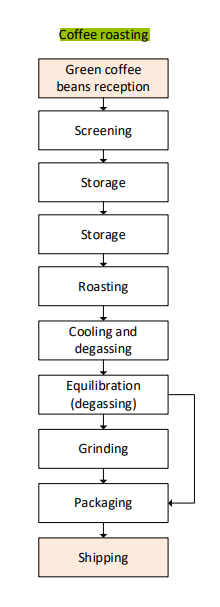
(FPE) Why do we roast coffee beans?
Simply because this is the tradition
(FQD) What is chain reversal
Chain reversal denotes a significant change in the food industry. Instead of production being dictated by available raw materials and producer decisions, consumer demand now heavily influences what products are offered in the market.
(FQD) Different ways of product development
Me-too products: same product, different brand
Line extensions: different version of same product, minimal change (e.g. coke zero)
Repositioning of existing products: Marketing > product, not much of a change in the product
New form of existing product: Different packaging (e.g. peperkoek on the go)
Reformulation of existing product: e.g. new recipe.
New packaging of existing products
Innovative products: More rigorous changes
Truly new products
(FCH) Techno-functional properties
The properties that the components have in the food.
e.g.
Giving colour, taste, preventing oxidation, giving structure to the food, stabilizing the food.
(FCH) pKa and pH
If the pH is above the pKa then the group will have dissociated.
For every dissociated group there is one H+ in the solution. Als this H+ will be titrated.
So no matter the pH, for every citric acid molecule three groups need to be titrated!!! (either the COOH group or a H+ in the solution)
(FCH) Minimum temperature for caramelization?
130 C
(FCH) What makes a reducing sugar a reducing sugar?
The carbon next to the oxygen atom in the ring has a free OH group. When the ring is open the sugar can react in reactions such as the caramelization or Maillard reaction
(FPH) Bilayers
Bilayers are formed by mono and diglycerides and are the main ingredients in a zero fat spread.
At room temperature, the bilayers are stiff and solid because the chains are relatively long
The bilayers are formed at higher temperatures where they are flexible
(FPH) Why does milk become less stable when the pH decreases?
Milk becomes less stable, and caseins too, since part of the CaPhosphates solubilizes, therefore diminishing the effective interactions that holds the casein sub micelles together. And hence below a pH of 5.3 all the casein will start to clump together.
(FPH) What is the skin on milk when heating milk?
Water evaporates, increasing the concentration of caseins and Calcium, inducing aggregations and thus skin formation.
One way out would be to put a lid on the pan to prevent water evaporation. Another way would be to foam the milk so that water evaporation is slowed down.
One reason why milk is overcooking is that the gas bubbles cannot be all released fast enough due to the formation of a skin, therefore pushing up the whole liquid, leading to spilling over.
Indeed, whipping while heating prevents it from spilling over.
(FPH) What does cream have less than milk
Whey proteins
Casein micelle
(FPH) Why is it better to whip milk under 7 degrees?
Because above 7 degrees the fat does not exhibit partial coalescence that efficiently since it is partially molten.
(FPH) Why is it better to add sugar after whipping?
Sugar tends to counteract membrane destabilization and thus counteract partial coalescence.
(FPH) Why does whipped cream become unstable after being whipped too harshly or too long?
When whipping you want to kind of coalesce the fat. Meaning you want fat droplets to kind of merge together but not all of the fat droplets becoming one.
When you whip too long this happens. The fat is coalesced completely. Individual fat droplets have merged together to form a single, larger mass of fat.
(FMB) Can all bacteria form spores?
No
(FMB) Spores
Not a universal trait among bacteria
If environmental conditions become unfavorable the vegetative bacterial cell initiates a process called sporulation to form a spore.
When they become a spore they don’t divide and don’t germinate
Bacteria and fungi have spores (spores in mold are hypha)
Bacteria spores are formed inside the cell.
Usually only gram positive bacteria make spores. But not all gram positive bacteria
(FMB) At which concentration (cfu/g or ml) bacterial spoilage becomes noticeable? Does this number also apply to yeasts?
For bacteria: 107, For yeast 106 (ml or cfu/g)
(FMB) What is a spoilage association?
The microorganisms that are well adapted to the product characteristics and that are the fastest growing population.
(FMB) Bacteria that grow on meat
Pseudomonas
Gram-negative
Aerobic
Can tolerate low temperatures
(FMB) Hurdle concept
A strategy in food preservation that combines multiple factors or hurdles to prevent microbial spoilage
(FPE) Scaling for time it takes for a substance to diffuse out a particle.
Coffee and brewing time: 𝑡 ∝ 𝑅2
t/t = (r/r)2
(FPE) Scaling for time it takes for a substance to percolate out a particle.
𝑡 ∝ 𝑅-2
This means smaller particles hinder water flow and increase percolation time because they create more resistance.
(FPE) Scaling relation between volume and radius
𝑉 ∝ 𝑅3
(FPE) Scaling relation surface area and radius
𝐴 ∝ 𝑅2
(FPE) making particles smaller calculations
Calculate change in volume (𝑉 ∝ 𝑅3) (23=8)
Calculate change in surface area (𝐴 ∝ 𝑅2) (22=4)
New volume/new surface area x how much smaller the particle has become. (8/4 × 2) (If particle is twice as small, then the same change in concentration is spread over a distance that is twice as small)
(FPH) Non-fat products
Zero-fat spreads are designed to have the texture and appearance of margarine but without the fat content.
They achieve this through a unique structure consisting of platelets stacked like a house of cards.
(FPH) DATEM
DATEM (diacetyl tartaric acid ester of monoglycerides), another ingredient in zero-fat spreads, is also incorporated into the bilayers.
The presence of DATEM, which has a charge, increases the repulsion between the bilayers, leading to a larger distance between them and contributing to the spread's overall rigidity.
The long hydrophobic chains of the mono and diglycerides make the platelets stiff at room temperature.
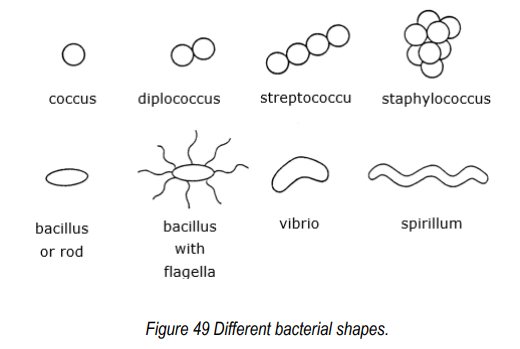
(FMB) Shapes of bacteria
(FMB) Enteros
Heat sensitive
Used as quality of heated products, if Enteros is present it suggest insufficient heating, recontamination or poor hygiene.
(FMB) Coliforms
Found in feces, fecal contamination
(FMB) Staphylococcus auereus
Indicates contamination through human handling
(FPH) Light scattering
Blue light scatters more than red light.
What are refined foods?
Proteins (whey, soy, …)
oils or fats (sunflower oil, olive oil, …)
Starch (binding agent, ….)
Sugar cubes
Processed and refined foods
Processed foods are made using non-refined production and the addition of ingredients.
(FQD) Steps in QACCP
define quality attributes - identifying key characteristics or properties of the product that are critical for its quality
Quantify critical values - Involves determining measurable target values for the quality attributes.
Define relevant parameters - Refers to identifying the important process parameters that influence the quality attributes during different production steps.
Measure the parameters - Refers to setting up methods to track and measure the defined parameters during the production process.
Define corrective measures: These are the steps that need to be taken when the actual values deviate from the target values during production.
Validation: Confirming that the settings, procedures, and corrective measures implemented are effective and lead to consistent achievement of the target values.
Documented: Highlights the importance of recording all the data and results throughout the process.
What is quality?
“Quality is meeting the expectations of the consumer”
The consumer is the starting point of thinking about ‘quality’
The term ‘expectations’ shows that the consumer does not consider fixed specifications.
Issues with defining quality
Wishes of the consumer can vary widely depending on differences in living conditions.
The average consumer can by no means judge all quality aspects.
The producer must take the quality requirements into account of other actors in the food supply chain.
What are the intrinsic quality attributes?
Sensory attributes (flavor, taste, appearance, texture, and smell)
Health attributes
Safety
Extrinsic quality attributes
Production attributes (Relates to consumer interest in production systems, e.g. paying more for organic products or products manufactured with concern for equitable income distribution, animal welfare or environmental considerations.)
Convenience attributes (Aspects of food that save time or energy that household members typically spend on shopping, food storage, food preparation, etc.)
Hazard Analysis Critical Control Point system (HACCP) consists of two main parts
A hazard analysis (HA), this is a method aimed at detecting microbial, physical, or chemical contamination risks along with all links of the chain.
Identifying and organiziing important control measures, Critical Control Points (CCP).
These points are set at production links where hazards are found. The CCps are meant as a precaution.
Quality Assurance Critical Control Points (QACCP) function
Controls all other quality parameters important in the food production chain.
Can standardize all batches of production; regardless of when a product is produced
Consumer insight, broken down into four.
Insight is non-obvious and does not come from just one source of information.
True insight need to be actionable.
Customer insights should be powerful enough that, when they are acted upon, they can persuade individuals to change their behavior.
To be sustainable, the goal of such customer change must be for mutual benefit.
How do you get consumer insights? (four ways)
Data quality - To understand and predict consumer behaviour, it is critical that from an almost infinitely large database only useful, high quality data is selected when attempting to gain consumer insights.
Analytics - Raw data needs to be analyzed to find buying behavior
Consumer research - Data does not show why a consumer shows a certain behavior. By interviewing and surveying consumers, a producers can gain a more complete understanding of the motivations of consumers and can use this to better forecast future buying behavior.
Database marketing - Translates these theories into a practical situation with a control and experimental group to test the insigths they have theorized.
Three possible claims in the world of packaging claims.
Marketing claims
Nutritional claims
Health claims
Marketing claims
Non-regulated
Don’t need scientific evidence
Should not indicate benefits to health or nutrition
E.g. Superfood, or grandma’s recipe.
Nutritional claims
Strictly regulated
Producer needs to prove that the product does indeed contain a certain amount of a certain nutrient.
Has absolute nutrient claims: Will state the product contains a certain % of the recommended daily intake for example
Comparative nutritional claims: Claims that state the chosen food product contains less of something than the ‘standard’ version of the product. e.g. low in salt, 30% less fat
Health claims
Are the heaviest regulated claims that require extensive scientific evidence before the claim can be made.
Three types:
Generic health claim: if product strengthens part of body or complements process in body. (e.g. helps digestion)
Disease risk reduction claim: Specifically focus on certain common diseases. Will state addition/reduction of an ingredient, followed by how it prevents disease. (e.g. lowers cholesterol - high cholesterol is a risk factor for heart disease)
Child development claim: Will claim that compounds in the food are beneficial during the growing of (young) children.
Time scale from idea to product
7-10 years on average
How long do products usually last on the market?
More than 20% of newly launched products dissappear within three years.
After five years less than 20% of the products are still on the market
Why food is processed?
Lots of food is harmful to eat unprocessed. (Grain, consumption of chaff can be dangerous)
You would not get a lot of nutrition out of it.
Minimal processing
a food production process that results in minimal changes to the structure and composition of the food
Functional foods
foods that provide health benefits beyond basic nutrition due to the presence of bioactive compounds that have a positive impact on bodily functions (e.g. yakult)
Meat production
Very inefficient because for 1 Kg of meat you need 5 to 8 kg of plant protein.
Traditional reasons for food processing
Preservation by heating and drying, and fermentation.
Removal or inactivation of toxic or ‘antinutrional’ compounds, by heating or removing ocmponents by dehydrating or rinsing, and in some cases treatment by heating, but also by treatment with bases or acids.
Newer, modern era motivations for food are:
Taste, cooking or baking creates flavors, may create a texture like a crust, and will make the food much tastier
Convenience, allowing a modern lifestyle by freeing up time for other activities than the preparation of food
Health, leading to milder conditions during processing, fortification of specific ‘functional’ foods.
Development of niche markets, such as lactose and gluten free foods
Sustainability and animal welfare; leading to foods that replace meat, and other foods that require many raw materials.
Characteristics of modern food processing.
Large facilities, more efficient, safer because food safety can be checked more easily.
Development of new formulations, new treatments, and new varieties of products is easier to do if you do it for a large quantity of products being made.
On a larger scale it is easier to be efficient in the use of resources and energy. (e.g. in dairy processing, the heat that is needed for pasteurization (heating to inactivate bacteria) is reused for more than 95%. In many other factories, heat is used repeatedly.
In large factories, you can use the raw materials more completely. Raw material that would otherwise be wasted can be used to make something else.
Tea processing
Tea leaves must be dried after picking to prevent spoilage and oxidation of the phenolic components in the tea
Drying can be done in different ways:
Steam: Steam will displace the oxygen in the air, and that will prevent oxidation. The green tea leaves will remain bright green, and the infusion will be strong.
Drying in open air: slowly rollling over the leaves with a weight. Opens the cells and vacuoles that have phenolic components and exposes them to oxygen and the enzyme polyphenol oxidase. Oxidation will make the leaves and the infusion a bit more yellowish and make the taste more ‘tea-like’.
Green tea processing (Japanese tea)
By steam
Displaces Oxygen in the air.
Will prevent oxidation of leaves
Leave will remain bright green, and the infusion will also be birght green and have a rather strong grassy flavor.
Chinese tea
Leaves dry in open air
Slowly rolling over the leaves with a weight
Opens the cells and vacuoles that have the phenolic components and exposes them to oxygen and the enzyme polyphenol oxidase, present in other parts of the plant cell.
The oxidation will make the leaves and the infusion a bit more yellowish and make the taste more ‘tea-like’.
White tea
Made from the still unopened leave buds, and these buds are only dried, so not rolled.
Oxidation has hardly proceeded, but since the leaves did not yet have that many phenolics in them, white tea is less green than the ‘normal’ green tea.
Black tea
oxidation of the phenolics in tea has been maximized.
Phenolics are green in their native state, but as they are exposed to oxygen, they become browner or even black.
Rollsing is used to crush the cells and bring the phenolics into contact with oxygen and polyphenol oxidase.
What are coffee beans made out of
Berries of coffea trees
Contain a lot of caffeine
Contain phenolic components
One berry has two coffee beans. Surrounded by a soft tissue.
Processing of berries from coffea trees
Drying and sieving to get rid of debris
Berries are crushed, and then te beans are soaked in water for a few days. Which softens the soft tissue around the bean.
Soft tissue is scrubbed/washed away.
Now beans are packed in bags.
Shipped and purchased by coffee roasters. Beans at this stage are green.
Roasting of coffee beans
Beans expand by about a factor of two and make beans very porous
Much of the phenolics is lost and some of the caffeine as well.
Proteins and sugars react to form maillard products, giving more taste and flavour to the coffee.
Beans are roasted and given time to degas.
Afterwards beans are packed either under vacuum or under nitrogen since oxygen reacts with many of the flavors.
Diffusion
Caused by brownian motion
At higher temperatures, molecules will move quicker.
Diffusion is faster if the concentration difference is larger and diffusion is faster if the distance is smaller.
What happens to the volume when the size of a coffee grind is reduced by two?
23 = 8
So a particle twice as small, would have 8 times as little coffee to be extracted from it.
This is based on the fact that the amount of coffee (or any substance) available for extraction is proportional to the volume of the grind. If the volume is reduced by a factor of 8, then the total amount of coffee available to be extracted from the particle is also reduced by the same factor, meaning there's 8 times less coffee available to diffuse into the surrounding liquid.
If it is a cube; generally, a particle twice as small, will have a surface area…
That is 4 times as small.
If we have 4 times less surface area, then we also will have 4 times less coffee coming out of the particle, all other things being equal.
What happens to the concentration gradient when we decrease the size of the coffee grinds by two?
When the coffee grinds are smaller, the distance that the coffee molecules need to travel to leave the grind and diffuse into the liquid is shorter.
This smaller distance makes the concentration change happen more abruptly, which increases the steepness of the concentration gradient.
A steeper concentration gradient means that the molecules will diffuse faster because the driving force for diffusion (the gradient) is stronger.
What if we want to remove 80% of coffee in the particle during brewing
Smaller particles: If you make coffee particles half their size, they have 4 times less surface area.
Less material: Smaller particles contain 8 times less coffee to extract.
Faster diffusion: Smaller particles create a steeper concentration gradient, so diffusion happens twice as fast.
Result: You’re extracting 8 times less coffee over 4 times less surface area, but with diffusion happening 2 times faster, so the overall extraction is 2 times quicker.
Disadvantages of making the particles smaller
The smaller the particle, the more surface area the particles share with the water and the slower the water will percolate through the grounds.
Hence the coffee ground cannot be too fine. The extraction would be fine, but the water would come out of the drip cone far too slowly.
Cold-brewed coffee
At lower temperatures molecules move more slowly and therefore diffusion is slower.
For cold brewing, the times are much longer: up to 10 hours.
Has fewer undissociated acids.
Pores in tea leaves
In coffee when coffee beans are roasted, a lot of gasses are produced which crack the coffee beans open and the resulting particles are also very porous.
In tea this is not the case, in tea the caffeine and other components have to diffuse through the cell walls which are very tought.
When tea is dissolved in water it will become a little porous but nowhere compared to the porosity of coffee grounds.
This also differs per tea, the rate of diffusion in black tea is about 1.4 times higher than the diffusion in the same components from green tea leaves.
Why do we not use a drip system for making tea?
Even when we would grind tea, it would require a longer time for extraction.
Since different molecules diffuse at different rates, just forcing the tea to be extracted faster would not result in good quality tea.
Quick coffee machines
Use concentrates
The coffee is brewed in a central factory on a very large scale, after which this coffee is concentrated.
This gives a concentrated liquid, or when all water is removed, a powder.
When making a cup of coffee, the vending machine only has to release a bit of the concentrate and a greater amount of hot water.
Extraction of coffee for liquid concentrates on a large scale
On an industrial scale, it is important that you use the coffee in the beans optimally.
On an industrial scale you want to get the most out of the raw material.
Because you need much fewer beans, because coffee beans are expensive.
Less waste, companies need a permit to produce waste and getting rid of the waste is difficult.
The process of extraction of coffee for concentrate
Coffee is ground into particles of 0.5 - 1.1 mm.
The ground coffee are put in 5-8 columns, water is led through these columns under pressure and at a temperature between 155 and 180 C.
The total contact time between coffee particles and water is long between 1.5 and 4 hours, to make sure that we get every last bit of coffee out of the grounds.
A lot more components move from the coffee particles to the water compared to coffee making at home.
The concentration of coffee components in the water becomes very high. The dry matter content reaches 25 to 27%, whereas in our homemade coffee, we usually have 1 to 3%
Removal of water from the concentrate using steam
Extract is led to a column that on the inside is equipped with a bundle of tubes.
The extract is divided over the inside of these tubes and runs across the wall as a thin continuous layer downwards.
At the same time, the column is made vacuum.
On the outer sides of the tubes, steam is applied.
Which gives condensation to the tubes and the condensation heat is transferred to the tubes.
The film of the extract on the inside of the tubes is heated and the water will evaporate.
Disadvantage: Flavoring components will evaporate.
Removal of water from the concentrate using freeze-concentrating
Water is not evaporated but frozen
Extract is led in a tube that is strongly cooled on the outside, for example with liquid nitrogen.
The water in the extract will freeze on the wall and this ice will only contain water
On the inside of the tube, a rotating blade scrapes the ice from the wall and small ice crystals accumulate in the extract.
This is called a scraped surface heat exchanger.
The extract is put in a vessel, where the ice crystal can grow into larger crystals
Then the ice is separated from the extract by a washing process.
Disadvantage: Expensive
Drying of concentrate using spray drying
most common way: spray drying
Concentrate is transferred to a high column and atomized (spread in small droplets)
Droplets fall down in the column, while warm air is fed in. The water in the droplets evaporates and is taken up by the air.
Eventually all water will be evaporated, and at the bottom, the droplets can be collected as small powder particles.
Drying of concentrate using freeze drying
Concentrate is frozen and then ground into small chunks.
These chunks are then spread out in a thin layer on large plates.
The plates are slid into a large vessel.
After this vessel is closed it is made vacuum, the pressure is so low, that the ice in the chunks is directly converted into vapor.
The ice sublimates
The water vapor is carried away to a condenser. The condenser contains a cooling devise that is so cold, that the water vapor condenses and freezes.
The chunks remain cold.
Scaling between time and particle size formula
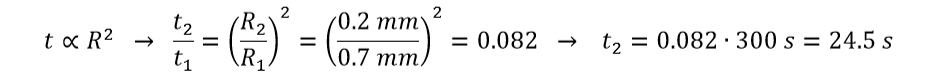
Function of mesoscopic physics
It acts as a bridge for formulating relationships between properties on a molecular scale and a macroscopic scale.
Categorizations (10 different ones)
Milk and dairy
Eggs & egg-based products
Meat
Fruits, vegetables and herbs
Grains and nuts
Bread and dough
Sauces
Confectionaries
Alcoholic beverages
Drinks and juices
Two issues with formulating relations between physical properties on a molecular scale and properties on a consumer relevant scale
Difference in lengthscale of a factor of a billion
Structural inhomogeneity
Mesoscopic structures
A structure that has a size between 1 nm and 1 mm
Colloidal systems
Consist of small particles dispersed in a continous medium
Something that falls in between a homogenous mixture and a heterogenous mixture
For example Fog is a colloid.
It consists of very tiny droplets of water dispersed in air
The dispersed phase are the water droplets
The continuous phase is the air where the water droplets are found
What type of flexible structure can result in the rigidity of its system with a low amount of mass?
Platelet-built structure
Order in different states of matter (solids)
Molecules are arranged in a fixed, regular pattern.
If you plot the number of molecules at these specific distances, you will observe "peaks" in a graph indicating that there are more molecules present at those discrete distances. The peaks reflect the structural regularity and periodicity typical of solid materials.
Order in different states of matter (liquids)
The arrangement is less rigid than in solids, yet there are still clusters of correlated molecules.
This limited extent of order indicates a more disordered and fluid molecular arrangement compared to solids. The peaks in a graph would still exist but would be much less pronounced and only noticeable over short distances, highlighting the transient nature of the interactions.
Order in different states of matter (gases)
There is no order, molecules are far apart from each other and move freely
In this state, you'd expect the graph depicting molecular distances to show no significant peaks, indicating a lack of consistent molecular arrangement or correlation at any distance
Relative humidity formula
p/pmax = RH
p=water vapour pressure
pmax=the maximum water vapour pressure
Water activity (aw)
Pproduct/Pmax
Pproduct=The water vapour pressure of the product
Pmax=The maximum water vapour pressure
Name 3 principle parameters that affect the physical properties of a system
Concentration, temperature, and pressure
Structure of milk
Fat globules, embedded in a fluid.
On a smaller scale one has casein micelles
These are built up of various proteins, among other constituents.
Whey proteins: These proteins, dispersed in the liquid surrounding the fat globules and casein micelles, contribute to the nutritional value and also play a role in the structure of dairy products.
Diameter of a fat globule
Range of 1 and 5 micrometers.
What is the thin shell of a fat globule made out of & what is its purpose?
Proteins
Phospholipids
Vitamin A
Cholesterol
These prevent the globules from coalescing together
Diameter casein micelle and what does it consist of?
Diameter is 0.1 microns
Consists of several types of protein aggregated together
Contains other consituents like calcium and phosphate ions, forming calcium phosphate complexes