please enter a deck name
1/36
Earn XP
Description and Tags
jejejeje
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
Ideal Gas
Negligible particle volume and no intermolecular forces. Valid at High Temperature and Low Pressure.
Real Gas
Non-ideal behavior occurs at Low Temperature and High Pressure where intermolecular forces and particle volume become significant.
Fundamental Property Relation
Connects internal energy to entropy and volume.
S, P
Natural variables of Enthalpy
V, T
Natural variables of Helmholtz Energy
P, T
Natural variables of Gibbs Energy
van der Waals (vdW)
The first cubic EOS. Introduces parameter $b$ (finite volume of particles) and $a$ (intermolecular attraction).
Soave-Redlich-Kwong (SRK) & Peng-Robinson (PR)
Improvements on vdW. PR is generally better for liquid density predictions.
Principle of Corresponding States
The theory that different fluids behave similarly at the same Reduced Temperature ($T_r$) and Reduced Pressure ($P_r$).
Compressibility Factor (Z)
If two fluids have the same $T_r$ and $P_r$, they have the same what factor?
Acentric Factor (w)
A measure of how non-spherical a molecule is (based on the shape of the molecule affecting its vapor pressure).
Virial EOS
A power series expansion (using coefficients B, C, D...). Excellent for gases, but generally valid only for the vapor phase (cannot model liquids).
Ion-Ion
strongest intermolecular force. Electrostatic attraction (Coulombic).
Ion-Dipole
Ion interacting with a polar molecule.
Dipole-Dipole
Interaction between two polar molecules.
Induction
A dipole inducing a momentary dipole in a non-polar molecule.
London Dispersion Forces (LDF)
Temporary fluctuations in electron distribution creating instantaneous dipoles. Present in all molecules (even noble gases).
Lennard-Jones Potential
A mathematical model for interaction energy.
Repulsion term
Dominates at very short distances (atoms crashing into each other).
Attraction term
Dominates at moderate distances (dispersion forces).
Residual Properties
The difference between a real fluid property and an ideal gas property at the same temperature and pressure.
MR = Mreal - Mideal
Used to calculate ΔH or ΔS for real fluids by taking a "path" through the ideal gas state.
zero
As Pressure approaches zero, the residual property approaches at what because all gases behave ideally at zero pressure
Isolated system
a criteria for equillibrium in which Maximize Entropy (S).
Closed Isothermal, Isochoric
a criteria for equillibrium in which Maximize helmholtz (A).
Closed Isothermal, Isobaric
a criteria for equillibrium in which Maximize Gibbs (G).
Thermal equilibrium
Tα = Tβ
Mechanical Equillibrium
Pα = Pβ
Chemical Equillibrium
Gα = Gβ
Thermal Stability
Stability Criteria in which Heat capacity must be positive
Mechanical Stability
Stability Criteria in which Pressure must increase when volume decreases and must be less than zero
Fugacity
It has units of pressure (Pa or bar) and behaves well at low pressures.
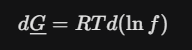
Formula of fugacity
Fugacity Coefficient
The ratio of "corrected pressure" to actual pressure.
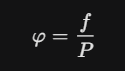
one (1)
For ideal gas, fugacity coefficient is at what value?
at P
For ideal gas, fugacity is at what value?
Equilibrium using Fugacity
fliquid = fvapor
Poynting Correction
Used to calculate the fugacity of a Compressed Liquid. It accounts for the effect of high pressure on the liquid phase (Pressure higher than saturation pressure).