Structure of atom class 11 NEET/CBSE
1/81
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
J.J Thomson
discovered electron through cathode ray tubes.
utilized Faraday’s study of electric discharge
found e/me value
Cathode Rays
move from (-) to (+) [cathode to anode]
they are invisibe
used a perforated anode (had holes in the anode) and a Phosphorescent Zinc sulphide coating behind the anode - that helped see the cathode rays when they hit the coating)
Travel in a straight line in the absence of a electric or magnetic field.
in the presence, cathode rays behave like (-) charged particles ———
electrons
The characteristic of cathode rays do not depend on the nature of gas used or the material of the elctrodes (e/me ratio is constant)
why are electrons the basic constituents of all atoms
Electrons mass and charge do not change even if emitted from various sources and various methods (e/me ratio remains the same)
e/me ratio
(electron charge) x (electron mass) = 1.7 × 1011
Millikan
through his oil drop experiment he found out electron charge.
electron charge value
-1.6 × 10-19 C
proton charge
1.6 × 10^-19 C
electron mass
9.1 × 10-31 kg
proton mass
1.672 × 10-27 Kg
neutron mass
1.674 × 10-27 Kg
Goldstein
Discovered protons
used a perforated cathode (cathode with holes in it)
anode rays emerge from the anode - a stream of (+) charged particles
Anode rays /canal rays
A stream of (+) charged particles
consisted of POSITIVE IONS
in case of H, the anode rays consisted of protons
H ——> H+ + e-
They travel in a striaght line
go from (+) to (-)
The properties of anode rays depend on the nature of gas. (e/m ratio is different for different gases)
The mass of positive particles is the same as the mass of the gas inside the tube
The magnitude of the positive charge depends on the number of electrons lost
A - 2e- ——→ A2+
A - 1e- ———> A1+
why do are the cathode ray tubes kept in very low pressure?
there is enough space at low pressure
allows gaseous atoms to accelerate at high speed
so when they strike each other they can eject electrons and create positive ions as well.
Chadwick
discovered neutron
bombarded a thin sheath of Be with alpha particles which emmitted electrically neutral particles
difference between alpha particles and He
Alpha - has 2 protons and 2 neutrons
He - has 2 protons, 2 neutrons and 2 electrons
Thomson’s atomic model
Plum pudding model
electrons are unevenly distributed in a sphere having positive charge
IMPORTANT FEATHURE - mass of atom is uniformly distributed over the whole atom.
FAILURE - due to Rutherford’s alpha scattering experiment
Rutherford
discovered nucleus
narrow beam of alpha particles pass through a lead plate and falls on a thin gold foil which has a circular zinc sulphide screen surrounding it
Results of alpha scattering experiment
Most alpha particles passed through the foil with no deflection - most of the atom consists of empty space
Few alpha particles deflected at small angles - positive charge is concentrated at a very small space
Very few of them deflected completely (180). - The (+) charged core is called nucleus
volume occupied by the nuclues is very small (10^-10) with the radius of atom being 10^-15)
Rutherfords nuclear model of atom
Nucleus - mass and (+) charge is centrally located in a small space
protons + neutrons = nucleons
the nucleus is surrounded by revolving electrons
resembled the solar system
drawbacks of Rutherfords Model
Could not account for the stability of an atom
Due to Maxwells electromagnetic theory which states that a charged particle with acceleration emits radiation and looses energy
an electron will loose energy and crash into the nucleus (it will take it less than 10-8 seconds )
Did not give any info about how electrons and distributed and the energy of these electrons
Atomic number (Z)
= number of protons = number of electrons in a neutral atom
Mass number (A)
Number of protons + number of neutrons
isotopes
same element, with different number of neutrons (different mass number)
Isobars
different elements with the same mass number
isotones
atoms with the same number of neutrons
isoelelectronic species
atoms that have the same number of electrons
Deuterium
an isotope of Hydrogen
has 1p 1e and 1n
developements leading to Bohrs model of atom
Dual character of electromagnetic radiation - both wave and particle like characters
Quantisation of energy
James Maxwell - wave nature of electromagnetic radiation
[Newton considered light as a stream of particles (corpuscles) but this concept could not explain the diffraction of light]
Electromagnetic radiations have a electric and magnetic field which are perpendicular to each other as well as perpendicular to direction of the propagation of wave
Do not need a medium to travel, can travel in vacuum . (speed = 3 × 108 m/s)
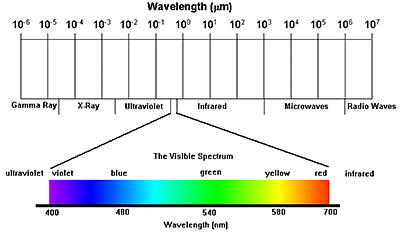
electromagnetic spectrum
visible region - 400nm to 750nm
consists of Violet, indigo, blue, green, yellow, orange, red
Cosmic rays < Gamma rays < X rays < Ultraviolet < visible light < Infrared < microwave < radiowaves
(in the order of increasing wavelength)
define wavelength
λ (lambda)
Wavelength is the distance between successive crests (or troughs) of a wave,
inversely related to frequency; as wavelength increases, frequency decreases, and vice versa.
define frequency (ν)
Frequency is the # of waves passing though a paticular point in one second
Wave number (ṽ)
1/λ
number of waves per meter
Amplitude
Height of crest or depth of trough
determines intensity/brightness/loudness of the light
Velocity ( c )
The distance travelled in one second by the wave
c=νλ
where c = 3 × 108
wave nature could explain
diffraction (bending of wave around a obstacle)
interference (combination of two waves to give a new wave)
wave nature could not explain
Black body radiation (radiation from hot bodies + why they change colour)
Photoelectric effect (ejection of electrons from a metal surface when striked with radiation)
Line spectra of atoms w.r.t. H
Variation of heat capacity of solids as a function of temperature
Plancks Quantum theory: particle nature of electromagnetic radiation
black body radiation
photoelectric effect
atomic spectra
line spectrum of hydrogen
black body radiation
Black body is an ideal body that emits and absorbs radiations of all frequencies.
No such body exists in reality
carbon black is the closest thing to a black body
the only factor on which the intensity and frequency of the emitted radiation depends is temp.
Max Planck
atoms and molecules could emit or absorb energy only in discrete quantities and not in a continuous manner.
Qauntum - smallest quantity of energy (it is called photon in case of light)
E=nhν
h = 6.626 × 10-34 Js (Plancks constant)
n → number of quanta
if we put c=λν into the equation,
E=nhc/λ
Photoelectric effect
electrons were ejected out of a metal surface when exposed to radiation
electrons were ejected out from the surface as soon as the beam of light hit - there is no time lag between the striking of light beam and the ejection of electrons
The number of electrons ejected ∝ intensity or brightness of light
DOES NOT DEPEND ON FREQUENCY
For each metal there is a threshold frequency - Min. amount of Energy required to eject electron from metal to overcome binding energy
binding energy=threshold energy
incident energy (Ei)
energy we put on an experiment
photoelectric equation
Ei = Eo + KE
Eo - threshold energy
can also be replaced by BE (binding energy)
BE=hνo=Work function
KE - kinetic energy of the electron when ejected out.
depends on the frequency of light
Only if Ei > Eo photoelectric effect occurs
hνi = hνo + ½ mev2
Evidence for the quantized electronic energy levels : atomic spectra
emission and absorption spectrum (line spectrums or discrete spectrums)
electron will always try to remain in the lowest energy level
experiment -
heat a gas
electron will jump to a higher energy level (energy absorbed) - absorption spectrum
electron will go back to its original energy level (energy released) - emission spectrum
continuous spectrum
VIBGYOR
has all the colours
spectroscopy
study of line spectrums
line spectrum
every element has its own unique line spectrum
fingerprints of atoms
line spectrum of hydrogen (will complete later)
Bohrs model of hydrogen and hydrogen like atoms
Postulates :
The electron moves around the nucleus in circular paths of fixed radius and energy
called orbits/stationary states/energy states
Energy of an electron does not change with time but
electron can move from n1 to n2 while absorbing energy
electron can move from n2 to n1 while releasing energy
Bohrs frequency rule
frequency of energy released from the transitions from 2. ^
ν = ΔE / h
ν = (E2-E1) / h
The angular momentum (L) of electron is quantised.
mvr=n x h/2π
m → mass of electron
v → velocity of electron
h → 6.626 × 10-34
n = 1,2,3,...
Meaning of n
n is just the numbering for the orbits/stationary states/ energy levels for electron
so the first energy state will be n=1
2nd energy state n=2 and so on
Radii of stationary states
rn= ao (n2/Z ) pm
ao = 52.9 pm → Bohr orbit, the radii of the first energy state for H
Z = atomic number for hydrogen like atoms
energy of stationary states
En= -RH (Z2/n2)
-RH → Rydbergs constant = -2.18 × 10-18 J = -13.6 eV/atom
energy for the ground state for H (n=1) is -RH
Why the negative sign in En= - RH (Z2/n2) ?
An electron's energy under an atom's influence is lower than the energy of an electron that is not under the influence of an atom (E=0). SO when the electron gets closer to the nucleus, its Energy gets lower (more negative)
a free electron’s energy is depicted as E=0
the energy of an electron at n=1 will be the lowest (most negative)
velocity of electron
V∝Z (positive charge on nucleus)
V∝ 1/n (principle quantum number)
V = 2.18 × 106Z / n m/s
energy gap between 2 orbits
ΔE=Ef - Ei
hc/λ = -RH(Z2/nf2) - - RH (Z2/ni2)
hc/λ = RHZ2 ( 1/ni2 - 1/nf2 )
Limitations of Bohr’s Model
Could not explain line spectra of multielectron systems
Could not explain ability of atoms to form molecules by chemical bonds
Failed to explain splitting of spectral line under the presence of a magnetic field (Zeeman effect) or an electric field (Stark effect)
could not account for the finer details in Hydrogen spectral lines. (Using sophisticated spectroscopic techniques it was observed that the spectral lines had doublets or triplets → closely spaces lines)
developments leading towards quantum mechanical model of atom
Dual behavior of matter
Heisenberg’s Uncertainty Principle
de-Broglie - dual behavior of matter
Just like radiation, matter should exhibit both particle and wave-like properties (dual behavior)
λ=h/p
derived from E=hν and E=mc2
hν=mc2
not significant for macroscopic objects
Heisenberg uncertainty principle
It is impossible to determine the exact position and the exact momentum of an electron simultaneously
Δx ⋅ Δp ≥ h/4π
Δx → error in position
Δp → error in momentum
Significance
rules out the existence of definite paths or trajectories of electrons and other similar particles
only significant for microscopic objects
quantum mechanical model
Based on quantum mechanics
developed by Heisenberg and Schrodinger
Schrodinger wave equation
Ψ (Psi)
does not carry any physical meaning
3D wave function
| Ψ | 2
probability density of finding electron at that point within an atom
maximum probability region is actually what is atomic orbital
What are the three quantum numbers that are solutions of the Schrödinger wave equation?
Principle quantum number (n)
Azimuthal quantum number (l)
Magnetic orbital quantum number (ml)
Which quantum number is not a solution to the Schrodinger wave equation?
electron spin quantum number (ms)
Principal quantum number (n)
represent which shell the electron is in
n=1,2,3,…
Determines the energy and size of an orbit
number of electron in a shell = 2n2
number of orbitals in a shell = n2
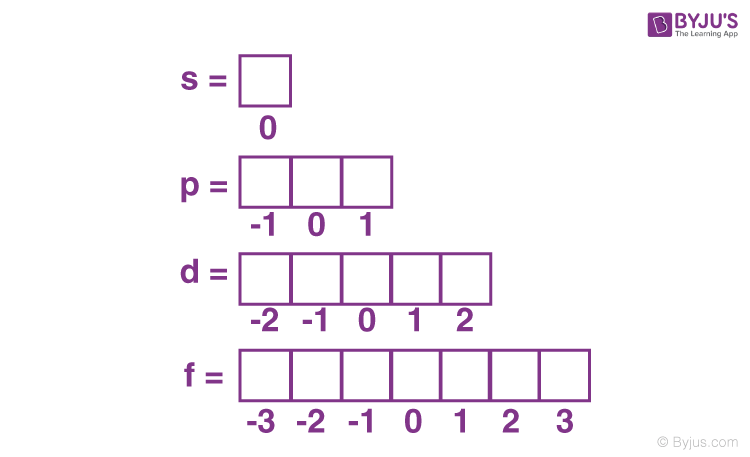
Azimuthal Quantum number (l)
also known as subsidiary quantum number
gives us ORBITAL angular momentum (in Bohrs postulates it was ORBIT angular momentum)
h/2π √l(l + 1)
gives 3D shape of the orbital
number of orbitals in a subshell = 2l + 1
number of subshells in a shell = n
0 → s
1 → p
2 → d
3 → f
Magnetic orbital quantum number (ml)
gives us total number of orbitals in a given subshell (l)
spatial orientation of the orbital w.r.t standard set of co-ordinate axis
explains Zeeman effect
fyi
orbit or shell → n
subshell → l
orbital → ml
electron spin quantum number (ms)
Tells us wether the electron is spinning clockwise or anticlockwise, explained the presence of two closely spaces lines in spectral lines (doublets)
+1/2 → clockwise
-1/2 → anticlockwise
an orbital cannot have more than two electrons and those two electrons must have opposite spins
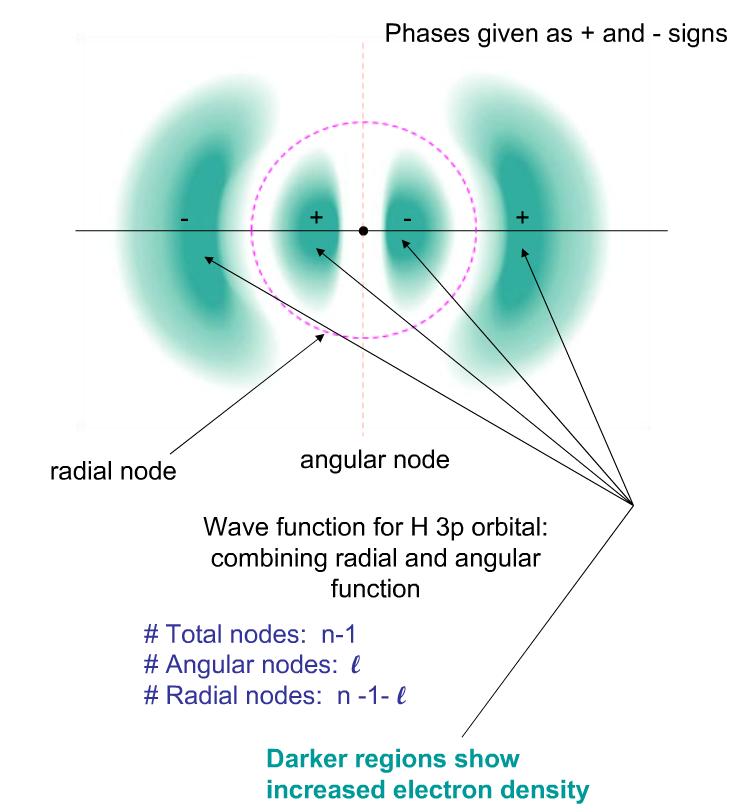
nodes
where probability of finding an electrons is 0
total nodes = n - l
Radial nodes
= n-1-l
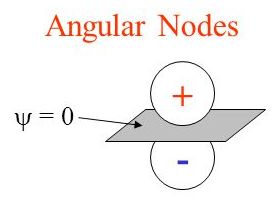
angular nodes
= l
shapes of atomic orbitals
s → spherical
p → dumb bell shaped
d → double dumbbell
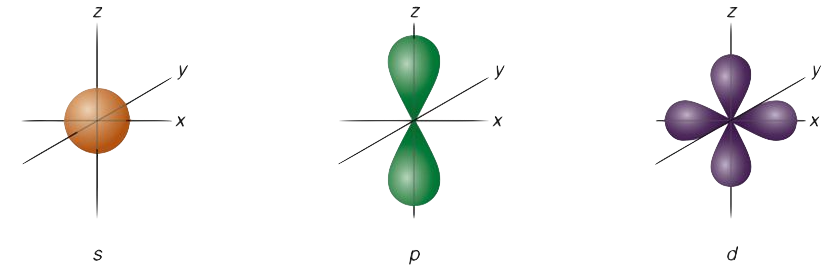
dz2 and dx2-y2 (degenerate d orbitals)
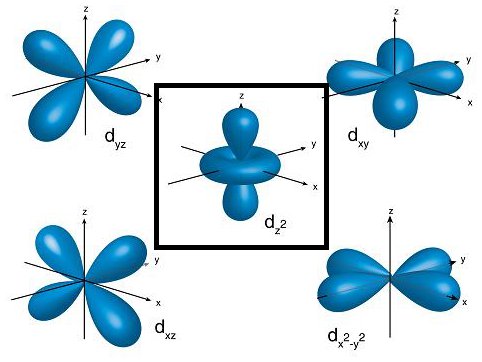
degenerate
orbitals having the same energy
energy of orbitals
the orbital with the lower (n+l) value has lower energy
if they have the same n+l value then the orbital having lower value of n has lower energy
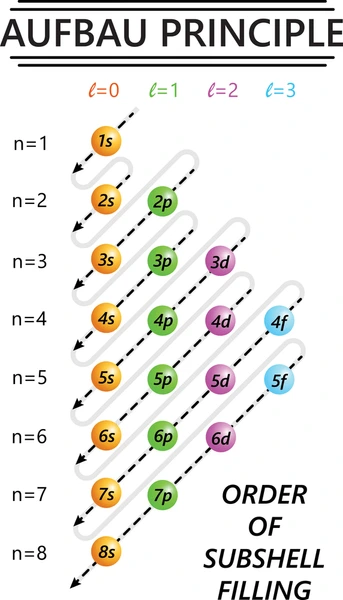
Aufbau Principle
electron filling takes place in order of increasing energy of subshells (from lower energy subshell → higher energy subshell)
note : when removing electrons from an atom, you remove it from the outermost subshell and you do not follow aufbau principle!
ex - 4s and 3d even tho 3d has higher energy and you would fill up 4s first, when removing electron you would remove from 4s and not 3d
Hund’s rule of maximum multiplicity
an electron will fill each orbital first before pairing together
because half filled (or) fully filled orbitals are highly stable
Pauli’s exclusion principle
no 2 electrons in an atom will have the same set of all 4 quantum numbers
electronic configuration of Chromium (Cr) - 24
[Ar]4s1, 3d5
this occurs because half filled oribitals are more stable
Cu - 29 E.C
[Ar] 4s1, 3d10