transplantation immunology
1/47
Earn XP
Description and Tags
week 9 immunology
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
cornerstones of organ transplantation
vascular anastomosis
short-term hypothermic organ preservation
inhibition of immune rejection
concept of acqquired immunologic tolerance
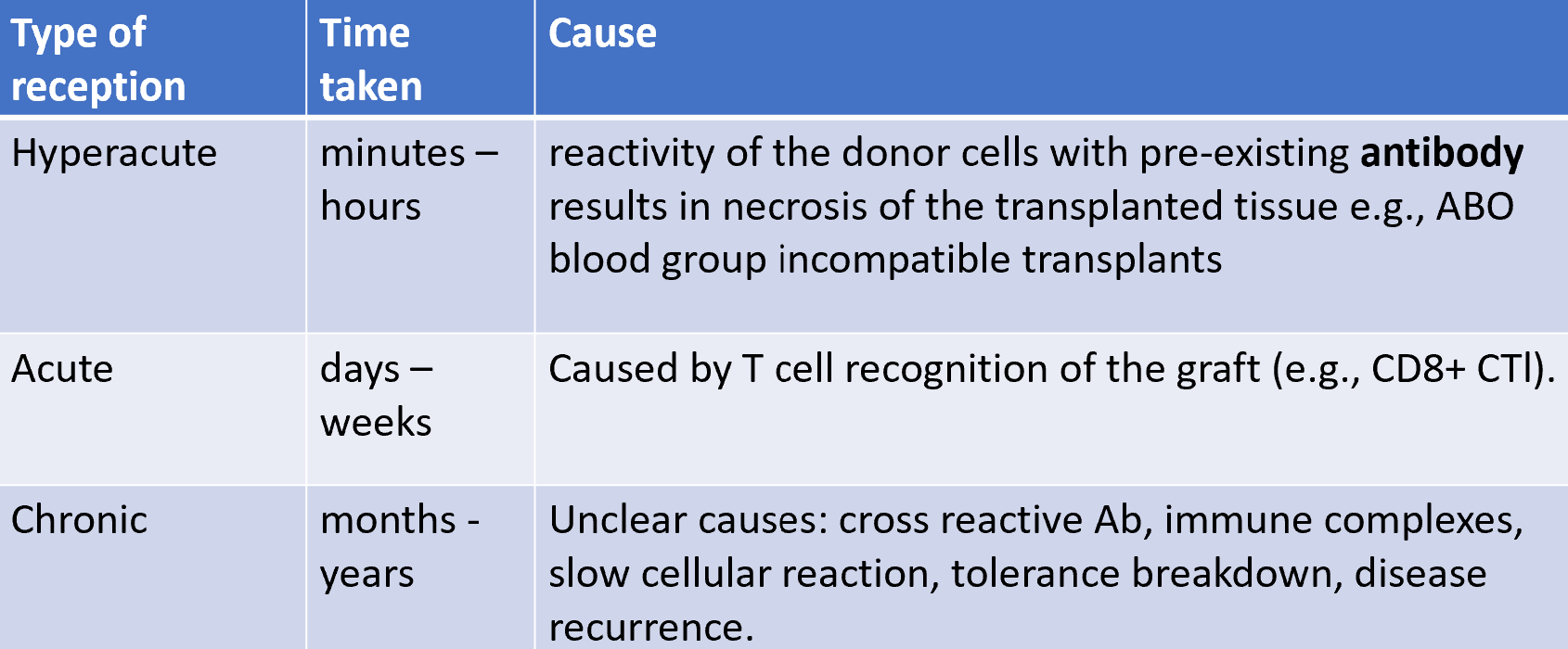
hyperacute, accelerated, acute and chronic rejection
medical and immunological considerations in transplantation immunology
acute and chronic graft rejection
immune signals
sensing of danger and stranger molecule
ischaemia and reperfusion
recognition of non self antigens
polymorphism of MHC genes
adaptive memory immunity and innate trained immunity
graft classification
autograft
allograft
isograft
xenograft
autograft
self-tissue transferred from one site to another in the same individual from one site to another in the same individual (usually accepted)
tissue transferred between different individuals in the same species: usually rejected unless given immunosuppressive therapy
allograft
tissue transferre between genetically different individuals in same species: usually rejected unless given immunosuppressive therapy
isograft
tissue transferred between genetically identical individuals: usually successful
xenograft
tissue transferred between different species
hyperacute rejection
better availability, easier to breed if animal organs are used
cardiac xenotransplantation
CRISPR
2022
survived for 2 months after transplant
possible problem:
organ rejection
anti-pig ABs may have attacked heart
pig virus
pt may have been too sick
graft acceptance and rejection depends on 2 factors
genetic relationship determines if graft is accepted
whether graft consists of isolated cells or tissue or an intact organ
autografts have low failure rate
cornea transplants common as anyone can donate them and they are privileged sites (not exposed to immune system)
autograft acceptance (skin graft)
day 1: grafted epidermis, blood vessels
days 3-7: revascularisation
days 7-10: healing
days 12-14: resolution
allograft rejection
rate of allograft rejection varies according to the tissue involved (skin grafts are rejected faster than other tissues)
first set rejection:
days 3-7: revascularisation
days 7-10: cellular infiltration
days 10-14: thrombosis and necrosis
second set rejection:
days 3-4: cellular infiltration
days 5-6: thrombosis and necrosis: immunologic memory
first set rejection: skin graft
day 1: grafted epidermis
days 3-7: revascularisation
days 7-10: cellular infiltration
days 10-14: thrombosis and necrosis
second set rejection: skin graft
day 1: grafted epidermis
days 3-4: cellualr infiltration
days 5-6: thrombosis and necrosis
graft rejection displays…
immunological memory
corneal allograft rejection
3 years status post penetrating keratoplasty
graft rejection mat transpire despite prophylactic efforts
skin graft rejection is the result of T cell mediated anti-graft response
grafts differing at the MHC are rejected at 10-13 days after grafting
naive mice that are given T cells from a sensitised donor behave as if they had already been grafted
immunological mechanism of a graft rejection
adaptive immune responses to a graft’s foreign proteins are the major barrier to effective tissue transplantation
mediated by either CD8+ or CD4+
antigens that differ between members of the same species are knowna s alloantigens → alloreactive response
different MHC alleles
major histocompatibility complex (MHC)
human leukocyte antigen (HLA) system in humans
histocompatibility antigens are encoded on more than 40 loci
most vigorous allograft rejection reactions are on MHC
3 major class I alleles:
HLA-A
HLA-B
HLA-C
3 major class II alleles:
HLA-DR
HLA-DQ
HLA-DP
polymorphisms in HLA, esp HLA-A, HLA-B and DR loci are most important biological barriers to a successful transplantation
as a closely HLA-matched graft is less likely to be recognised and rejected, HLA mismatching has a substantial impact on graft survival
major and minor histocompatibility molecules serve as alloantigens in graft rejection
even complete matching at the MHC doesn’t ensure graft survival
although synergic grafts are not rejected, MHC identical grafts from donors (that differ at other loci- minor H antigen loci) are rejected
rejected more slowly than MHC disparate grafts
minor histocompatibility antigens
if a polymorphic protein differs between the graft donor and recipient, can give rtise to an antigenic peptide
can be recognised by recipient’s T cell as non self and elicit an immune response
such antigens are minor H antigens
mechanisms of rejection
immune response to a transplanted organ consists of both cellular (lymphocyte mediated) and humoral (AB mediated) mechanisms
T cells are central in graft rejection
rejection reaction consists of sensitisation stage and effector stage
direct mode of recognition
indirect mode of recognition
recognition by AB
direct and indirect pathways of allorecognition contribute to graft rejection
direct allorecognition
organ grafts carry with them APCs of donor origin/passenger leukocytes
APCs leave graft and migrate to secondary lymphoid tissue of recipient where they activate host T cells that bear corresponding TCRs
associated with acute rejection
indirect allorecognition
uptake of allogeneic proteins by recipient’s own APCs and their presentation to T cells by self MHC molecules
peptides derived from both foreign MHC molecules themselves and minor histocompatibility antigens can be presented by indirect allorecognition
ABs are produced against non self antigens from same species and are known as alloABs
ABs in graft rejection
pre-existing alloABs against blood group antigens and polymorphic MHC antigens can cause rejection of within minutes of transplantation
ABs react with antigens on vascular endothelial cells of the graft and initiate complement and blood clotting cascades
vessels fo the graft become blocked, causing its rapid destruction
to reduce graft rejection
ABO matching
HLA tissue typing
cross matching
types of graft rejection
sensitisation stage
CD4 and CD8 T cells recognise alloantigen expressed on cells of foreign graft (including major and minor histocompatibility alloantigens)
→ Th cell activation by APCs (dendritic cells)
‘passenger leukocytes’: population of donor APCs that migrate from graft to lymph node, express allogeneic MHC antigens of the donor graft
direct vs indirect pathway of allorecognition, each leading to generation of different sets of allospecific T cell clones
effector stage
hallmark of graft rejection involving cell-mediated reactions is an influx of T cells and macrophages into graft
recognition of foreign class I alloantigens of the graft by host CD8+ cells can lead to CTL-mediated killing
in some cases, CD4+ T cells that function as class II MHC-restricted cytotoxic cells mediate graft rejection
cytokines secreted by Th cells play a central role
effector mechanisms involved in graft rejection
IL-2, IFN-gamma, TNF-beta are important mediators in graft rejection
IFN-gamma promotes the influx of macrophages into graft and their subsequent activation into more destructive cells
TNF-beta has a direct cytotoxic effect on cells of a graft
during rejection episode, cytokine levels increase
mechanisms of target cell destruction
direct killing by Tc cells and indirect tissue damage through release of cytokines such as IFNγ and TNF from Th-1
direct killing by NK cells enhanced by interferon
attack by AB dependent cellular cytotoxicity (ADCC)
phagocytosis of target coated with AB (heightened by bound C3b)
sticking of plts to AB bound to the surface of graft vascular endothelium leading to microthrombi formation
complement mediated cytotoxicity
macrophages activated non-specifically by agents such as IFNγ and possibly C3b can be cytotoxic for graft cells, perhaps through extracellular action of TNF and 02 radicals generated at cell surface
graft versus host disease (GvHD)
T cells recognise alloantigens in recipient tissues
mature T cells of graft are immunocompetent, reactive cells
frequently occurs following HSC transplantation (bone marrow, cord blood)
can often be ameliorated by removing T cells from donor bone marrow before transplantation
GVH pathogenesis
involves secretion of IL-1β, TNF and IFN-γ from damaged host tissue
both donor and recipient dendritic cells activate donor Th1 cells to secrete IL-2 and more IFN-γ
host is attacked by donor CTls and NKs (Fas-FaL perforin/granzyme B pathways) inducing apoptosis
Treg cells may be harnessed to limit GVH
GvH disease in humans: donor cells react against recipient
GvHD can affect many different parts of the body
skin, eyes, stomach and intestines are affected most often
can range from mild to life threatening
chronic GVH: has a somewhat good prognosis if ilimited to skina and liver, not if multiple organs are involved
graft-versus leukaemic effect (GVL)
mild to moderate GVHD can also be beneficial when donor immune cells attack recipient tumour cells that have surivived the aggressive chemo and radiation
in case of leukaemia: graft versus leukaemia
though removing donor T cells from graft reduces risk of GVHD, this may not be best approach for marrow transplants used in antineoplastic therapy
foetus is an allograft that is tolerated
high likelihood that mother and father have different HLA types
foetus expresses on HLA haplotype of maternal and one of paternal origin
paternal HLA class I and class II molecules expressed by foetus are alloantigens
foetus protected from pre-existing alloreactive ABs or T cells
no sign of immunological rejection if mother has more than one child with same father
foetus as an allograft
maternal blood with immunocompetent lymphocytes circulate in contact with foetal trophoblast
foetomaternal tolerance
trophoblast doesn’t express MHC class I and II
nutrient depletion reduces T cell responsiveness
cytokine milieu (TGF-β, IL-10) suppresses development fo effector T cells in favour of iTreg cells
stromal cells of maternal uterine tissue that directly interacts with placenta represses local expression of key T cell attracting chemokines
blood typing
blood type O is considered universal donor
blood type AB is called universal recipient because they can receive an organ or blood from people with any blood type
HLA tissue typing
phenotypic methods
serology (microcytoxicity)
tissue typing: mixed lymphocyte reaction
phenotypic methods have been phased out and replaced with molecular methods based on DNA analysis
genotypic methods
PCR based techniques for detecting HLA genes
PCR-RFLP (restriction fragment length polymorphism)
variable number tandem repeat (VNTR) typing
short tandem repeat typing
HLA typing by microcytotoxicity assay
peripheral WBC of donor and recipient are incubated with allele-specific ABs and with complement
lysis of cells bearing antigen is assessed by addition of specific dyes
HLA typing by mixed lymphocyte reaction
irradiated stimualtor lymphocytes from donor are incubated with responding T cells from recipient
proliferation of recipient T cells is measured by uptake into cell DNA and indicates degree of compatibility
crossmatching- complement dependent cytotoxicity
cell based assay
can determine presence of donor specific anti-HLA ABs in serum of recipient
B and T cells are separately tested against serum from recipient
serum from recipient is added to donor lymphocytes (T or B) in presence of complement
negative test: donor specific anti-HLA ABs are absent, no complement activation
positive test: donor specific ABs bind to lymphocytes, complement activationa and cell lysis
crossmatching: flow cytometry
cell based
donor lymphocytes are mixed with recipient’s serum
lymphocytes bind to donor specific ABs and are quantified by flow cytometer
quantification:
measurement of the fluorescence intensity as a ratio of control
serial dilutoions of recipient’s serum are made to react with donor lymphocytes and the minimum dilution which yield’s a negative result gives a measurable estimate
virtual crossmatching
bead technology beads are impregnated with different ratio of 2 fluorochromes resulting in a signal that is unique to specific bead
serum HLA ABs will react with HLA antigens on bead
beads are washed and incubated with a second AB
immunosuppression therapy
induction therapy: immunosuppression is started at the time of transplantation to prevent an immune response against the graft
anti-t CELL ABs and/or IL-2 receptor antagonists
maintenance therapy: transplant recipients usually need to be maintained on immunosuppressive drugs for the rest of their lives
calc inhibitors, purine metabolism inhibitors and mTOR inhibitors are used, often together with steroids
treatment of rejection episodes:
humoral rejection can be treated with IV Ig, plasmapheresis and anti CD20 AB
variety of immunosuppressive anti-T cell agents are also commonly employed
mode of action of immunosuppressants
rabbit anti-thymoglobulin and anti-CD52 monoclonal ABs are used to deplete T cells and other leukocytes before transplantation
anti-CD3 monoclonal AB prevents generation of signalling by T cell receptor complex
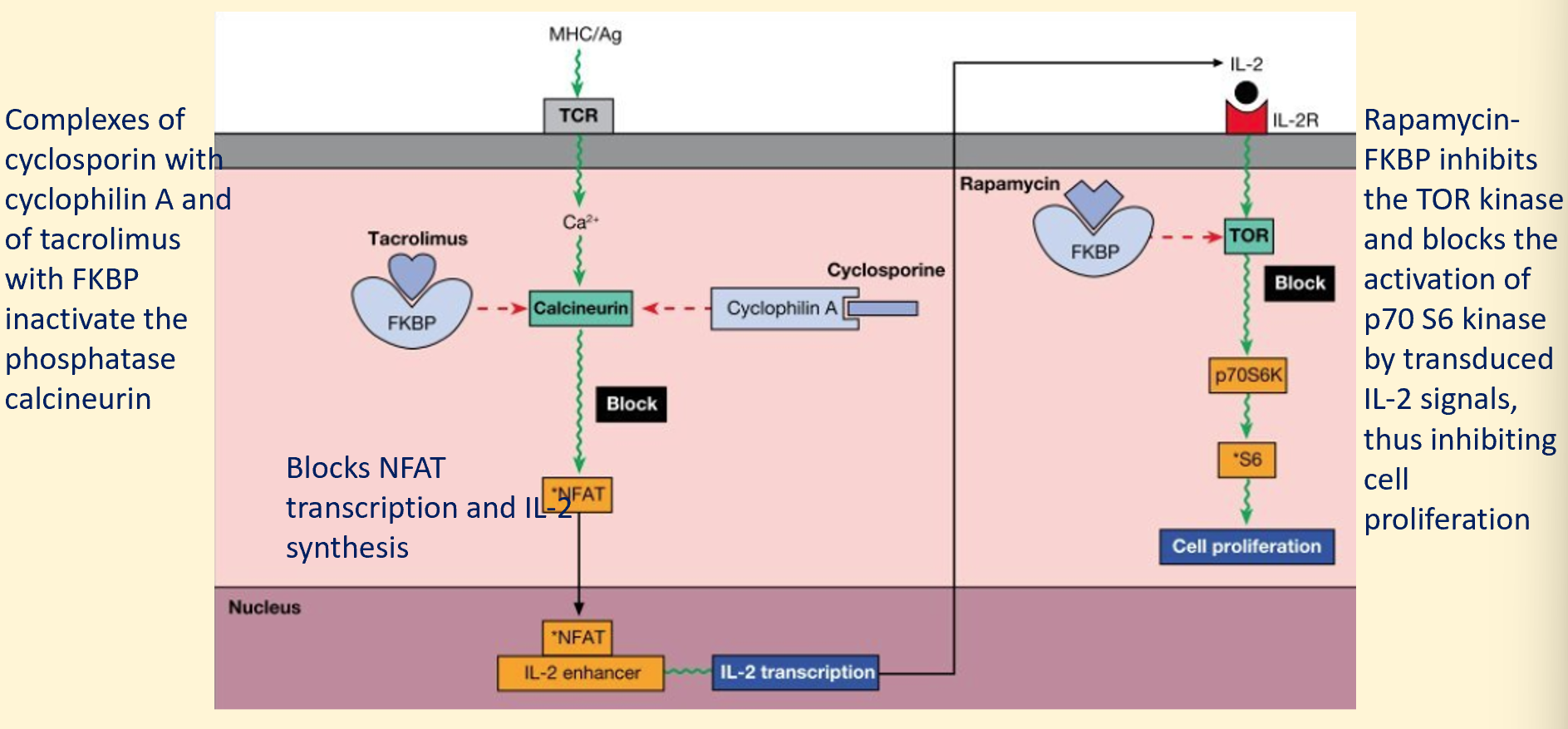
cyclosporin and tacromilus interfere with translocation of nuclear factor of activated T cells to nucleus by inhibiting calcineurin
CTLA-4-Fc fusion protein belatacept binds B7 and prevents generation of co-stimualtion via CD28
anti-CD25 AB binds to high affinity IL-2 receptor on partially activated T cells and prevents IL-2 signalling
sirolumus interferes with activation of the mTOR cascade which is required for differentiation of effector T cells
cyclosporin and rapamycin act at different stages of T cell activation
costimulatory blockage: inducing tolerance
T cell activation requires co-stimulatory signals (engagement of CD28 on the T cell surface by the B7 molecules on surface of APC)
CTLA-4 binds to CD80-86 with higher affinity than CD28 and therefore soluble CTLA-4-Ig fusion protein blocks these co-stimulatory signals, resulting in T cell anergy
monoclonal AB to CD40L on T cell would block co stimulatory signals normally provided by CD40 on the APC
stem cell therapy
creating an ideal transplant entirely from cells of the recipient would eliminate the need for immumosuppression
possible to isolate stem cells from various adult organs (incl bone marrow)
application in medicine
privileged sites
transplants at certain anatomical sites are generally accepted without any immune rejection
absence of lymphatic drainage is probably critical common factor
haemapoietic stem cell transplants (bone marrow transplant)
3 sources of stem cells are used, listed in order of decreasing mature T cell contamination:
peripheral blood (enriched by cytokine administration)
bone marrow
cord blood