Chem summer work
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Hypothesis structure
If/then/because
Independent variable
what is being changed
Dependant variable
what is being measured
Constant
Is not changed throughout all trials
When making a graph make sure to?
LABEL
Matter
is any object that has mass and takes up space
Solid
Particles are close together and vibrate in place, and has a fixed shape and volume
Liquid
Particles are slightly farther apart and can move past one another, has a fixed volume but takes the shape of the container
Gas
Particles are far apart and can move freely, making the gas have neither a fixed volume or shape.
Chemical properties of matter
A substances ability to experience a chemical reaction that changes it into another substance.
Homogeneous Mixtures
Mixtures that are blended evenly and are unable to distinguish individual substances.
Heterogeneous Mixtures
Mixtures that are not evenly distributed and can see each thing with your eye.
0 degees celcius = how many Kelvin
273
KHDBdcm
King henry died by drinking chocolate milk.
D IS ANYTHING UNIT STARTING WITH DA
B- grams, meters, liters
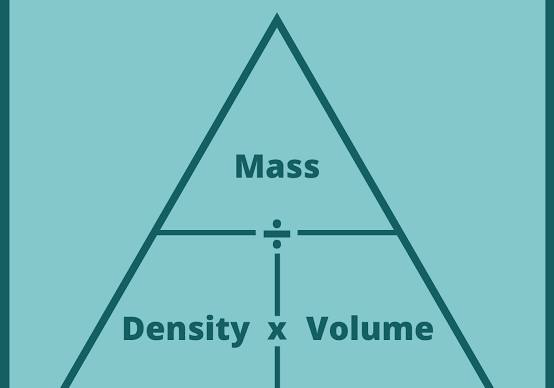
Use density pyramid for conversions.