Chemistry - Topic 1 - Atomic Structure
1/56
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
relative isotopic mass
the mass of an atom of a particular isotope relative to 1/12 the mass of a carbon-12 atom
common simple molecular structures
H2 - hydrogen
N2 - nitrogen
O2 - oxygen
F2 - fluorine
Cl2 - chlorine
Br2 - bromine
I2 - iodine
P4 - phosphorous
S8 - sulphur
what does mass spectometry do?
separates ions based on their mass-to-charge-ratio (m/z)
in mass spectometry, the bigger the mass…
the less deflection for a given force (a=F/m)
first step of mass spectometry
vaporisation
using high temperature
or low pressure (to prevent thermal decomposition)
in what form are the ions shot by the electron gun (cathode ray) and what is the name of this process
ionisation
all elements are made into ions which have a +1 charge
what generates the force exerted on the ions in mass spectometry
a strong electromagnet (which creates a magnetic field) + (force depends on charge of ion)
what can mass spectometry NOT do?
diffrentiate between ions that share the same m/z ratio
what process happens in mass spectometry and write a balanced equation with state symbols for this process involving a monatomic atom.
ionisation
X(g) + e- → X+(g) + 2e-
what process happens in mass spectometry and write a balanced equation with state symbols for this process involving a diatomic element.
ionisation
½N2(g) + e- → N+(g) + 2e-
how to calculate m/z ratio
atomic mass/charge
mass spectrum of a diatomic element e.g. chlorine. why are there two clusters of peaks?
because there are both monatomic and diatomic ions hitting the detector
chlorine is diatomic; initially diatomic ions are formed but some of them fragment, forming monatomic ions (and neutral atoms which are not detected)
why is it not possible to predict the relative heights of the two clusters of peaks compared to each other?
it is difficult to predict how much fragmentation will occur.
which factors affect the forces of attraction between oppositely charged particles?
the distance between them (bigger means weaker attraction)
the charges on them (bigger charges means stronger attraction)
first ionisation energy definition
the energy required to remove one electron from each atom in one mole of gaseous atoms (forming one mole of gaseous 1+ cations)
what is the unit to measure e.g. first ionisation energy?
kJ/mol
write an equation for the first ionisation energy of helium.
He(g) → He+(g) + e-
write an equation for the second ionisation energy of helium.
He+(g) → He2+(g) + e-
second ionisation energy definition
the energy require to remove on electron from each 1+ cation in one mole of gaseous 1+ cations (forming one mole of gaseous 2+ cations)
is removing an electron from an atom an endothermic or exothermic process?
endothermic, because energy is absorbed to overcome the electrostatic attraction
which is larger: the first or second ionisation energy of helium? why?
second ionisation energy of helium because there is a greater net attraction to the nucleus because there is less repulsion from other electrons.
why does helium not have a third ionisation energy?
as helium only has 2 electrons.
relative atomic mass
the weighted average mass of an atom of an element relative to 1/12 the mass of an atom of the carbon-12 isotope
describe what happens, in terms of electronic transitions. during an atomic emission spectrum
electrons in atoms gain/absorb energy (from heat or high voltage) and move up (are excited) to a higher energy level
electrons drop back down to a lower energy level, releasing energy in the form of a photon as they do so
why are only specific frequencies (or wavelengths) of light given off during an atomic emission spectrum?
the difference in energy between two energy levels is ΔE
the frequency of a photon is directly proportional to this energy gap (E=hf)
specific frequencies of light (rather than a continuous spectrum) are given off because:
energy levels are quantised
so there are only certain energy gaps between them
so certain freqencies of light cannot be emitted / only certain frequencies
how do atomic emission spectra provide evidence for the existence of quantum shells?
only specific frequencies of light are given off (when electrons are excited)
frequency of a photon is proportional to its energy (delta E = h (Plank’s constant) * f). the energy of the photon matches the energy gap (of the transition it has just undergone) therefore there are only certain energy gaps. this means the electron can only have certain amounts of energy (its energy levels are quantised)
shielding definition
an effect of inner electrons which reduces the pull of the nucleus on the electrons in the outer shell of an atom (due to repulsion between the inner and outer shell electrons)
thanks to shielding, the electrons in the outer shell are attracted by an 'effective nuclear charge' which is less than the full charge on the nucleus
how to explain large jumps between successive ionisation energies?
e.g.
there is a large jump between the 2nd and 3rd ionisation energies
this indicates that the third electron has a much greater net attraction to the nucleus (more than expected if it was in the same shell)
this indicates the third electron is being removed from a shell that is closer to the nucleus, where it experiences much less shielding* (because there are fewer shells between this shell and the nucleus)
why is it difficult to plot values of e.g. successive ionisation energies of sodium on a graph?
the range of values is too large! can’t pick a suitable scale.
name approporiate axes to a plot a graph to depict successive ionisation energies.
log ionisation energy against e.g. electrons removed
explain why the graph takes this shape: a general increase in ionisation energy with a couple of big jumps.
general increase is due to reduced electron-electron repulsion due to fewer electrons being present as they are sequentially removed (so greater net attraction to the nucleus)
big jump between 1st and 2nd, indicates second electron is being removed from a shell that is closer to the nucleus (less shielding)
big jump between 9th and 10th, indicates tenth electron is being removed from a shell that is even closer to the nucleus (even less shielding, actually no shielding!)
NB: this indicates the second shell can be occupied by eight electrons
which factors affect the net electrostatic force of attraction between an electron and a nucleus in an atom?
shielding (depends on the number of core elctrons, hence this depends on which shell the electron is in)
nuclear charge (depends on number of protons)
distance between the electron and the nucleus (depends on which shell the electron is in)
general trends of ionisation energy
general increase in first ionisation energy across a period
general decrease in first ionisation energy down a group
what is periodicity?
a repeating pattern across different periods.
orbital definition
a region within an atom where there is a high chance of finding an electron (and that can hold up to two electrons with opposite spins)
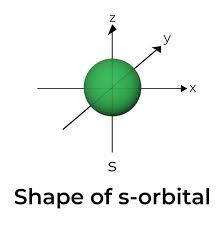
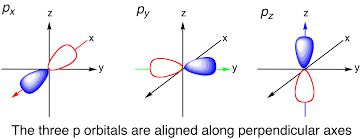
how do s and p orbitals differ?
by shape, energy levels and direction in space (point and direction)
shells are divided into…
subshells, which all have similar energy
subshells are further divided into…
orbitals, which all have identical energy
what is the probability of finding an electron within an orbital?
95%
which d block elements are exceptions in terms of electron configurations and why?
chromium and copper as some in 3d but only one in 4s as the 3d subshell is more stable when it is half or completely full.
what three factors does ionisation energy depend on?
nuclear charge
extent of shielding (dominated by the number of core electrons/inner shells)
the orbital from which the electron is being removed (relates to the distance between electron and nucleus)
these factors also affect the atomic radius (distance between outer electron and nucleus)
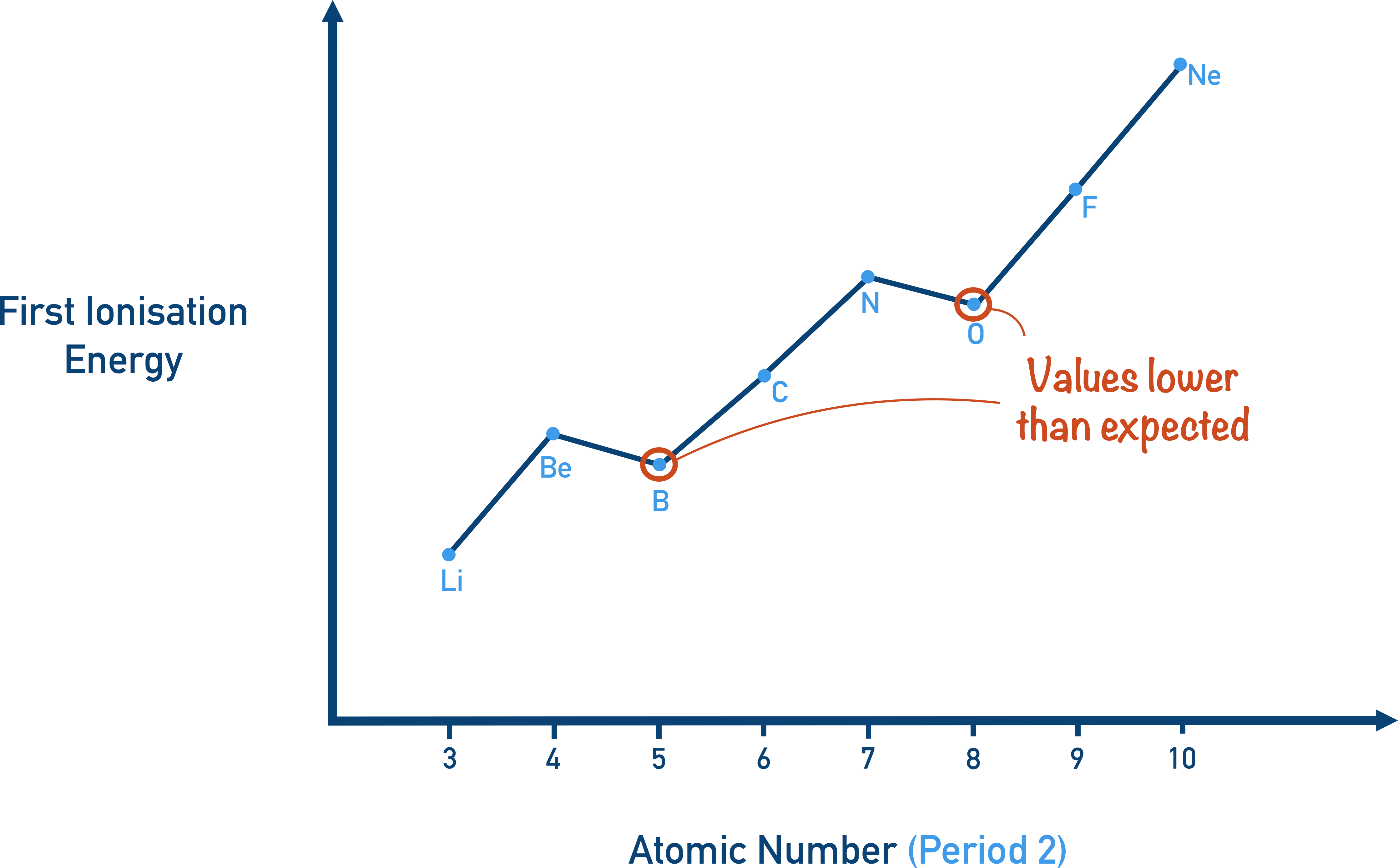
why is there a drop going from He to Li?
in Li, the electron is removed from the 2s subshell (a 2s orbital). in He, it is removed from the 1s subshell.
the electron in 2s subshell in Li is further from the nucleus and more shielded so, despite the increased nuclear charge of Li, it is less attracted to the nucleus, so less energy is required to remove it.
why is there a drop going from N to O?
the electron in oxygen is being removed from a doubly occupied orbital, in which there is more repulsion between the electrons
this decreases the net nuclear attraction which causes the slight drop in ionisation energy
explain why ionisation energy decreases down a group
nuclear charge increases, which leads to greater nuclear attraction
there are more shells, so there is a greater shielding effect (as there are more inner shells), which leads to a decreased nuclear attraction
the electron is being removed from a higher energy shell which is further from the nucleus, which leads to a decreased nuclear attraction
the latter two effects outweigh the increased nuclear charge, so the nuclear attraction becomes weaker down the group, so ionisation energy decreases down the group.
explain why there is a general increase in ionisation energy across a period
as the period is crossed, nuclear charge increases but there are the same number of occupied shells so shielding remains approximately constant so net nuclear attraction increases
(this means atomic radius decreases and ionisation energy increases across a period)
what causes the decrease in first ionisation energy moving from group 2 to group 3?
despite the extra proton in Al, the electron is removed from a 3p orbital instead of a 3s orbital
the 3p orbital is slightly shielded by the 3s orbital which decreases the net nuclear attraction (i.e. the 3p orbital is higher in energy than the 3s) and causes the slight drop in ionisation energy
what is an isotope?
isotopes are ATOMS of the same element with the same number of protons but a different number of neutrons
draw a diagram showing the general trend in first ionisation energy across a period.
draw an s-orbital
draw a p-orbital
remember to only draw one if asked for p-orbital, and 3 if asked for p subshell
how many electrons are there in the following subshells: s, p and d?
s subshell - 2 electrons
p subshell - 6 electrons
d subshell - 10 electrons
consider the elements Al, Si, P and S.
the first ionisation energy increases as you go across the period from Al to P.
explain why sulphur does not follow this trend. (2)
sulphur has 4 electrons in the 3p sub-shell AND one 3p orbital is doubly filled
so there is a (slight) repulsion between the electrons in the same 3p orbital (resulting in a lower first ionisation energy)
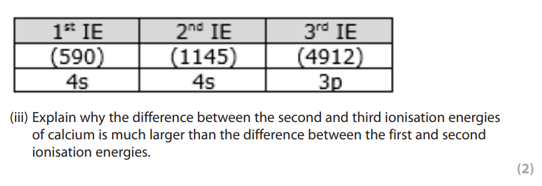
why is there a large increase betewen the second and third ionisations of calcium?
Ca: [Ar] 4s2
removing the third electron from a different shell at a lower energy level which is closer to the nucleus so the nuclear attraction is greater, much more energy required to overcome
why is there no large increase within the first four ionisation energies of vanadium?
V: [Ar] 4s2 3d3
3d and 4s subshells are close in energy/similar distance from the nucleus
so all four electrons experience a similar attraction to the nucleus
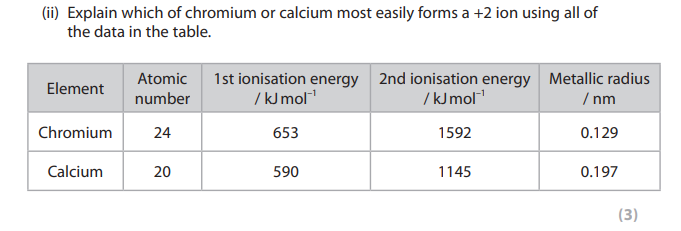
would you expect a calcium ion to be bigger, smaller or the same size as a calcium atom? give TWO reasons to explain your answer. (2)
smaller
because Ca2+ has one less (sub) shell of electrons
and the radtio of protons: electrons has increased/more protons than electrons/greater effective nuclear charge