ENGI-105 Thermodynamics Final Exam Flash Cards
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
122 Terms
What is the DEFINITION of Entropy?
The INABILITY to do WORK
What is the 2nd Law of Thermodynamics (general, simple version)?
Entropy is ALWAYS Increasing
Alt. Version - The Inability to do Work (Entropy) is Always Increasing
What is the Kelvin-Plank statement for the Second Law of Thermodynamics?
It is IMPOSSIBLE to constructed a device which will operate in a CYCLE AND produce NO EFFECT other than (work) and the exchange of HEAT with a SINGLE RESERVOIR
- You CAN Convert Work -> Heat with 100% Efficiency
Ẇ -> Q̇, η = 100%
- You CANNOT convert Heat -> Work with 100% Efficiency
Q̇ -> Ẇ, η ≠ 100%
(mnemonic - Kelvin-Plank = 2 people, 2 people = 2 reservoirs)
What is the Clausius statement for the Second Law of Thermodynamics?
It is IMPOSSIBLE to construct a device which operates in a CYCLE and produce NO EFFECT other than the TRANSFER OF HEAT from a COOLER body to a HOTTER body
(mnemonic - plug in your refrigerator!)
What is Minimum Loss?
Work and Efficiency are Maximized
What is Irreversibility?
A process that CANNOT return to its original state ; the level of irreversibility is quantified by the property of entropy
T or F: Entropy is NOT Conserved, it's Generated
TRUE
~WHAT ARE THE 3 WAYS ENTROPY IS GENERATED?~
1. Friction
2. Unrestrained Expansion
3. Heat Transfer through Finite Temperature Differences
T or F: Specific Entropy is a Dependent Property
FALSE
ex. Ice = Low Entropy
Water = More Entropy
Steam = Lost of Entropy
What are the Gibb's Equation for Entropy?
T(ds) = du + P(dv)
T(ds) = dh - v(dP)
where
h = specific enthalpy = u + Pv
v = specific Volumes
s = specific entropy
u = specific internal energy
T = Temperature (in KELVIN)
P = Absolute Pressure (NOT GAUGE)
What is the Equation for entropy quantified in terms of Temperature (T) and Specific Volume (v)?
s(T_2, v_2) - s(T_1, v_1) = (C_v)ln[T_2/T_1] + (R)ln[v_2/v_1]
What is the Equation for entropy quantified in terms of Temperature (T) and Pressure (P)?
s(T_2, P_2) - s(T_1, P_1) = (C_p)ln[T_2 / T_1] - (R)ln[P_2 / P_1]
What is the Equation for Entropy of a RESERVOIR?
ds = dh / T OR S = Q / T
where
s = Specific Entropy (kJ/kg * K)
S = entropy (kJ/K)
T = Temp. (in KELVIN)
What are the conditions of an ISENTROPIC Process?
s = constant, therefore
1. Adiabatic (No heat is transferred, Q=0)
2. Internally Reversible
~ISENTROPIC RELATIONSHIP EQUATION - Temperature (T) and Pressure (P)~
(T_2 / T_1) = (P_2 / P_1)^[(k-1)/k]
Where
T = KELVIN
P = Absolute Pressure
k = C_p / C_v (dependent on type of gas molecule)
NOTE - T_2 and P_2 are both in the NUMERATOR, respectively
~ISENTROPIC RELATIONSHIP EQUATION - Temperature (T) and Specific Volume (v)~
(T_2 / T_1) = (v_1 / v_2)^[k-1]
Where
T = KELVIN
v = specific Volume
k = C_p / C_v (dependent on type of gas molecule)
NOTE - T_2 is in the NUMERATOR, and v_2 is in the DENOMINATOR, respectively
~ISENTROPIC RELATIONSHIP EQUATION - Pressure (P) and Specific Volume (v)~
(P_2 / P_1) = (v_1 / v_2)^[k]
Where
P = Absolute Pressure
v = specific Volume
k = C_p / C_v (dependent on type of gas molecule)
NOTE - P_2 is in the NUMERATOR, and v_2 is in the DENOMINATOR, respectively
T or F: You can ONLY use the isentropic relationship equations for T, v, and P if the SPECIFIC HEAT CONSTANTS (C_p and C_v) are CONSTANT
TRUE - at least for the equations I wrote above
What kind of relationship is Isentropic Efficiency?
it is a Work over Work Relationship (W / W) where the larger of the works (either Isentropic Work [W_s] or Actual Work [W_a]) is in the denominator
~ISENTROPIC EFFICENCY - TURBINE~
η_s,T = W_a / W_s = (h_3 - h_4a) / (h_3 - h_4s)
where
W_a = Actual Work
W_s = Isentropic Work
h = specific enthalpy
~ISENTROPIC EFFICENCY - COMPRESSOR~
η_s,C = W_s / W_a = (h_2s - h_1) / (h_2a - h_1)
where
W_a = Actual Work
W_s = Isentropic Work
h = specific enthalpy
~ISENTROPIC EFFICENCY - NOZZLES~
η_s,N = KE_a / KE_s = (v_a)^2 / (v_s)^2
where
KE_a = Actual Kinetic Energy
KE_s = Isentropic Kinetic Energy
v_a = Actual Velocity
v_s = Isentropic Velocity
~ISENTROPIC EFFICENCY - PUMP~
η_s,P = W_s / W_a = (vΔP) / (h_2a - h_1) = (h_2s - h_1) / (h_2a - h_1)
where
W_a = Actual Work
W_s = Isentropic Work
h = specific enthalpy
v = specific volume
P = pressure
T or F: the isentropic work of a pump equation is W = Δu = (vΔP)
FALSE: W = Δh = (vΔP)
~CARNOT HEAT ENGINE EFFICIENCY~
η_max = 1 - (T_L / T_H)
where
T must be KELVIN
~CARNOT REFIGERATION COP~
β_max = T_L / (T_H - T_L)
where
T must be KELVIN
~ENTROPY OF A PROOCESS EQUATION~
ds/dt = ΣQ̇/T + Σ(ṁs)_in - Σ(ṁs)_out + σ
where
T = Temperature
σ = generated entropy
ṁ = mass flow
What are the 5 Thermodynamic Cycles that have powered and modernized society?
1. Rankine Cycle
2. Refrigeration Cycle
3. Brayton Cycle
4. Otto Cycle
5. Deisel Cycle
What is a Perpetual Motion Machine of the 1st kind (PPM-1)?
It is A hypothetical device that produces more energy (work) than it consumes, essentially creating energy from nothing, thus violating the First Law of Thermodynamics (Conservation of Energy).
What is a Perpetual Motion Machine of the 2nd kind (PPM-2)?
It is a hypothetical device that violates the Second Law of Thermodynamics by spontaneously converting heat from a single thermal source entirely into useful work, achieving 100% efficiency without any heat rejection to a colder sink, essentially cooling its surroundings to absolute zero and generating perpetual energy.
For a Proposed Device, It's suggested efficiency is HIGHER than that Cycles Maximum/Isentropic Efficiency.
What is the Efficiency equation for a Heat Engine?
η = 1 - (Q_L / Q_H)
How do you derive the Carnot Heat Engine Efficiency equation from the real Heat Engine?
When you add entropy to the heat engine drawing you have:
S_H = entropy leaving the hot reservoir and entering the cycle, and S_H = Q_H / T_H
S_L = entropy leaving the cycle and entering the cold reservoir, and S_L = Q_L / T_L
S_gen = generated entropy entering the cycle
where you can equate the entropy's as:
S_H + S_gen = S_L
BUT, because this is a Carnot Cycle, we are assuming S_gen = 0, therefore:
S_H = S_L -> Q_H / T_H = Q_L / T_L
-> Q_L / Q_H = T_L / T_H
and since the efficiency of a heat engine is:
η = 1 - (Q_L / Q_H)
The Carnot efficient of a Heat Engine is:
η = 1 - (T_L / T_H)
where
T must be KELVIN
(similar processes can he done for the other components, but I just did this one for clarity)
~WHAT ARE 4 PROCESSES IN THE CARNOT CYCLE?~
ISENTROPIC Compression
ISOTHERMAL Heat Addition
ISENTROPIC Expansion
ISOTHERMAL Heat Rejection
What is the MINIMUM ΔT required to have a reasonable enough Heat Transfer?
ΔT = 10
What are the Air Standard Cycles?
1. Brayton Cycle
2. Otto Cycle
3. Deisel Cycle
When comparing actual and isentropic efficiency/COPs, how do they relate for Irreversible, Reversible, and Impossible Processes?
Irreversible: η_actual < η_max , β_actual < β_max
Reversible: η_actual = η_max , β_actual = β_max
Impossible: η_actual > η_max , β_actual > β_max
What is Cogeneration?
Cogeneration = Generating Power and Water Heat (Q_L), with 100% theoretical efficiency
~WHAT ARE 4 PROCESSES AND COMPONENTS IN THE RANKINE CYCLE?~
1 -> 2 : ISENTROPIC Compression (PUMP)
2 -> 3 : ISOBARIC Heat Addition (BOILER)
3 -> 4 : ISENTROPIC Expansion (TURBINE)
4 -> 1 : ISOBARIC Heat Rejection (CONDENSER)
What should the state of the working fluid be at each state/processes?
state 1 : must be Liquid
state 2 : must be Liquid
state 3 : must be Superheated Vapor
state 4 : must have Quality better than 0.85
If at a certain state in a Rankine Cycle, it has a certain percent moisture (for example 15%), what does this mean?
At that state, the Quality is x = 0.85
(basically, turn the moisture percentage into a decimal, and subtract it from 1 to get the quality at that state:
ex. 1 - 0.15 = 0.85 -> x = 0.85)
What equations derived from the 1st law is associated with the 4 Processes of the Rankine Cycle?
1 -> 2 : (PUMP) : Ẇ_pump = ṁ(h_2 - h_1) [For isentropic = vdP]
2 -> 3 : (BOILER) : Q̇_in = ṁ(h_3 - h_2)
3 -> 4 : (TURBINE) : Ẇ_Turb = ṁ(h_3 - h_4)
4 -> 1 : (CONDENSER) : Q̇_out = ṁ(h_4 - h_1)
~THERMAL EFFICENCY EQUATION OF A RANKINE CYCLE~
η = Ẇ_net / Q̇_in = (Ẇ_T - Ẇ_P) / (Q̇_in)
where
Ẇ_T = Work of Turbine 1
Ẇ_P = Work of the Pump
What is the Shaft Power of a Steam Piston Equation?
Shaft Power = τ*ω
where
τ = Torque
ω = Angular Velocity
Why do fan blades sizes increase as steam flows through a Turbine?
Because the vapor is expanding, so the size of the blades increases to compensate for the increase in volume and obtain the maximum steam power, as well as decrease fan damage over time.
What losses occur at the Pipes, Turbine, Pump, and Condenser of the Simple, REAL Rankine Cycle?
Piping Losses = Frictional Losses reduce the available energy content of the stream
Turbine Losses = Turbine isentropic (or adiabatic) efficiency
Pump Losses = Pump isentropic (or adiabatic) efficiency
Condenser Losses = relatively small losses that result from cooling the condensate below the saturation temperature in the condenser
HOW DO YOU INCREASE RANKINE CYCLE EFFICIENCY? (Hint: there are 4 main ways)
1. INCREASE Boiler Temperature and Pressure (Material Constraint)
2. DECREASE Condesner Pressure (becomes more two-phase, as well as low quality)
-> INCREASE Moisture in Turbine OUTLET
-> LARGE Turbines required as density of steams drops
3. Reheat (adds to Q_in, but also Low Pressures)
4. Preheat using an Open or Closed Feedwater Heater
(Short Hand - INCREASE Temperature of Injecting Heat, DECREASE Temperature of Ejecting Heat)
What is a Reheat Rankine Cycle?
Reheat is adding a second turbine stage to reheat steam after partial expansion in a high-pressure turbine, increasing thermal efficiency and preventing excessive moisture in the low-pressure turbine.
The steam expands in a high-pressure turbine, then returns to the boiler for reheating before expanding further in a low-pressure turbine, boosting work output and efficiency
~THERMAL EFFICENCY EQUATION OF A REHEAT RANKINE CYCLE~
η = Ẇ_net / Q̇_in = (Ẇ_T1 + Ẇ_T2 - Ẇ_P) / (Q̇_Boiler + Q̇_reheat)
where
Ẇ_T1 = Work of Turbine 1
Ẇ_T2 = Work of Turbine 2
Ẇ_P = Work of the Pump
What equations derived from the 1st law is associated with the 4 Processes of the REHEAT Rankine Cycle? (its helps to draw or look up the reheat cycle first)
(PUMP) : Ẇ_pump = ṁ(h_2 - h_1) [For isentropic = vdP]
(BOILER) : Q̇_in = ṁ[(h_3 - h_2) + (h_5 - h_4)]
(TURBINE) : Ẇ_Turb = ṁ[(h_3 - h_4) + (h_5 - h_6)]
(CONDENSER) : Q̇_out = ṁ(h_6 - h_1)
What is an OPEN Feedwater Heat Exchanger?
A direct-contact mixing chamber that mixes extracted steam from a turbine with cooler feedwater, heating the water
T or F: In and Open Feedwater Heat Exchangers, it requires 2 pumps, one before the heater, and one after
TRUE
~THERMAL EFFICENCY EQUATION OF A OPEN FEEDWATER HEAT EXCHANGERS RANKINE CYCLE~
η = Ẇ_net / Q̇_in = [Ẇ_T - (Ẇ_P1 + Ẇ_P2)] / (Q̇_in)
where
Ẇ_T = Work of the Turbine
Ẇ_P1 = Work of Pump 1
Ẇ_P2 = Work of Pump 2
What equations derived from the 1st law is associated with the 4 Processes of the OPEN FEEDWATER HEAT EXCHANGER Rankine Cycle? (its helps to draw or look up the cycle first)
(PUMP 1) : Ẇ_P1 = (1-y)ṁ(h_2 - h_1) [For isentropic = vdP]
(PUMP 2) : Ẇ_P2 = ṁ(h_4 - h_3) [For isentropic = vdP]
(BOILER) : Q̇_in = ṁ(h_5 - h_4)
(TURBINE) : Ẇ_Turb = ṁ(h_5 - h_6) + (1-y)ṁ(h_6 - h_7)
(CONDENSER) : Q̇_out = (1-y)ṁ(h_7 - h_1)
y = (h_3 - h_2) / (h_6 - h_2)
NOTES -
- ṁ_3 = ṁ_2 + ṁ_6, continuity equation, they are mixing, and because they are mixing, the pressures MUST be equal on these lines
- line 3 MUST be liquid because it's going to go through pump 2 and same goes for lines 1 & 2 for Pump 1
- line 6 will be two-phase or superheated vapor
What does MAXIMUM PREHEAT Imply for an Open Feedwater Heat Exchanger?
Quality (x) = 0
What is an CLOSED Feedwater Heat Exchanger?
It is used to preheat boiler feedwater using extracted steam from a turbine, improving efficiency by transferring heat indirectly without mixing the water and steam streams, keeping them at different pressures
T or F: In and Closed Feedwater Heat Exchanger also requires 2 pumps, one before the heater, and one after
FALSE : It only needs ONE Pump before the Heat Exchanger, BUT it requires a TRAP after the heater from the excess steam turbine line used to heat the feedwater because the pressure needs to lower before mixing in the condenser
What is a Trap?
A trap chokes flow, unrestrained expansion (aka high entropy generator), isenthalpic valve that drops pressure
~THERMAL EFFICENCY EQUATION OF A CLOSED FEEDWATER HEAT EXCHANGERS RANKINE CYCLE~
η = Ẇ_net / Q̇_in = [Ẇ_T - Ẇ_P] / (Q̇_in)
where
Ẇ_T = Work of the Turbine
Ẇ_P = Work of the Pump
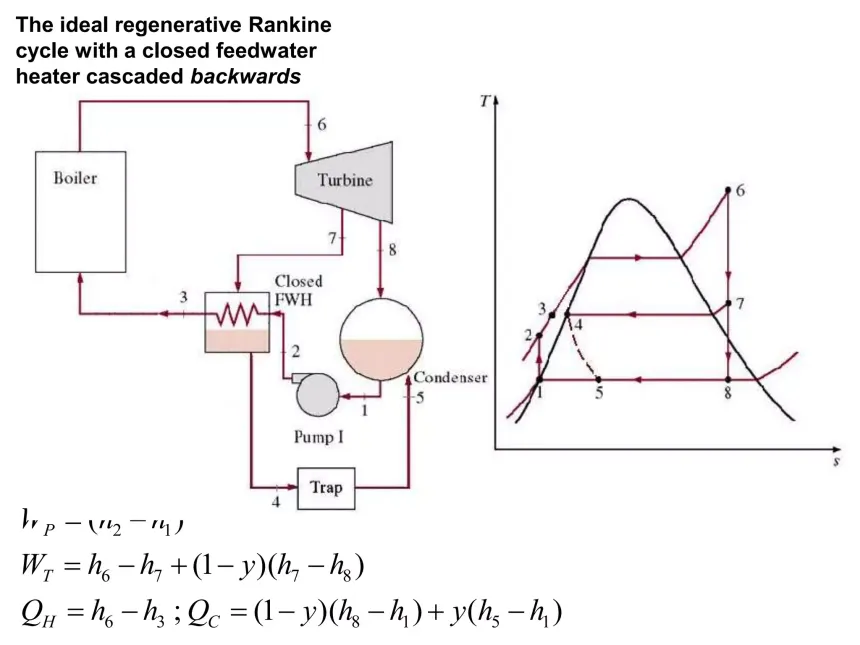
What equations derived from the 1st law is associated with the 4 Processes of the CLOSED FEEDWATER HEAT EXCHANGER Rankine Cycle? (its helps to draw or look up the cycle first)
(PUMP) : Ẇ_P = ṁ(h_2 - h_1) [For isentropic = vdP]
(BOILER) : Q̇_in = ṁ(h_4 - h_3)
(TURBINE) : Ẇ_Turb = ṁ(h_4 - h_5) + (1-y)ṁ(h_5 - h_6)
(TRAP) : ṁ_7 = ṁ_8 -> h_7 = h_8
(CONDENSER) : Q̇_out = TBD
NOTES -
- Line 3 could be two-phase or superheated vapor
- ALL the energy that was in lines 5 -> 7 MUST go to
2 -> 3
Between a Closed and Open Feedwater Heat Exchanger, which yields a higher Efficiency, and why?
Closed feedwater heater, because there is no mixing, so higher temperature differences through heat exchange can happen.
In what situation is a Closed Feedwater Heat Exchanger needed over a Open one?
For a Car Radiator, you do NOT want to mix Air with EthyleenGlycol
What TWO things sustain a Pressure Difference?
Walls & Shock Waves
What are the main Paid Sources of Fuel to optimize efficiency?
Coal, Oil, and Natural Gas
What are the main Non-Paid Sources of Fuel, where efficiency doesn't really need to be optimized for cost?
Geothermal (Low Temp = Loe Efficiency), Nuclear (Low Temp = Low Efficiency), Solar (Low Flux)
REDUCED PRESSURE RELATIONSHIP (for air)
(P_r1 / P_r2) = (P_1 / P_2)
where
r = relative, this corresponds with entropy
USE TABLE A-22! The 'P_r' values correspond to a certain Temperature in KELVIN
RELATIVE SPECIFIC VOLUME RELATIONSHIP FOR AIR
(v_r1 / v_r2) = (v_1 / v_2)
where
r = relative, this corresponds with entropy
USE TABLE A-22! The 'P_r' values correspond to a certain Temperature in KELVIN
What did George Brayton envision?
He envisioned continuous heat addition at constant pressure for a reciprocating engine. This eventually morphed into a compressor, followed by burner, and followed by an expansion device ; Gas Turbine!!
What are significant qualities of a Gas Turbine?
- Its Efficiency is Lower than Otto and Deisel
- Requires High BackWork
- BUT, It has a SIGINIgANTLY HIGH Power Density
~BACK WORK RATIO - BRAYTON CYCLE~
BWR = Ẇ_C / Ẇ_T
Ẇ_C = Compressor Work
Ẇ_T = Turbine Work
What is the working fluid of the Brayton Cycle?
Air (C.A.S.A. assumed for Brayton Cycle)
~WHAT ARE 4 PROCESSES AND COMPONENTS IN THE (CLOSED) BRAYTON CYCLE?~
1 -> 2 : ISENTROPIC Compression (COMPRESSOR)
2 -> 3 : ISOBARIC Heat Addition (COMBUSTER)
3 -> 4 : ISENTROPIC Expansion (TURBINE)
4 -> 1 : ISOBARIC Heat Rejection (HEAT EXCHANGER)
What is different for the Open Brayton Cycle than the Closed one?
It's not actual a cycle, it uses the atmosphere are the Heat Exchanger
~WHAT ARE THE 5 AIR STANDARD ASSUMPTIONS?~
1. The Working Fluid is Air, and it acts as an Ideal Gas
2. All Processes are Internally Reversible
3. Combustion Process is replaced by Heat Addition
4. Exhaust Process is replaced by Heat Rejection
5. C.A.S.A. [Cold Air Standard Assumption] - Properties of Air and Specific Heat Values are Constant at 25C
What is the 'k' value of Air assumed under the ASA?
k = 1.4
~PRESSURE RATIO EQUATION~
r_p = (P_1 / P_2) = (P_3 / P-4)
P = Pressures at states 1, 2, 3, and 4, respectively
~THERMAL EFFICENCY EQUATION OF A BRAYTON CYCLE~
η = Ẇ_net / Q̇_in = [Ẇ_T - Ẇ_C] /Q̇_in
ALT EQ
η = 1 - [1 / (r_p)^[(k-1) / k]]
where
Ẇ_T = Turbine Work
Ẇ_C = Compressor Work
r_p = Pressure Ratio
What equations derived from the 1st law is associated with the 4 Processes of the Brayton Cycle?
1 -> 2 : (COMPRESSOR) : Ẇ_Comp = ṁ(h_2 - h_1) = ṁC_p(T_2 - T_1)
2 -> 3 : (COMBUSTER) : Q̇_in = ṁ(h_3 - h_2) = ṁC_p(T_3 - T_2)
3 -> 4 : (TURBINE) : Ẇ_Turb = ṁ(h_3 - h_4) = ṁC_p(T_3 - T_4)
4 -> 1 : (HEAT EXCHANGER) : Q̇_out = ṁ(h_4 - h_1) = ṁC_p(T_4 - T_1)
NOTE - C_p equations in this format can ONLY be used for CONSTANT C_p, but since we usually assume ASA's for a Brayton Cycle, we can apply it here
What are Combined Cycles?
A Combined Cycle is two or more thermodynamic cycles operating from a single heat source
Typically, the waste heat from one cycle is used as the heat source for another
This boosts both total power output and overall efficiency
~TOTAL EFFICENCY OF A COMBINED CYCLE EQUATION~
η_tot = η_top + η_bottom - (η_top * η_bottom)
T or F: Brayton Cycles are exclusively as Toping Cycles in Power Plants, with Rankine Cycles acting as Bottoming
TRUE
What is the definition of Specially Separated?
It is where different stages of a cycle, or different components, are isolated from one another in
space within a larger system or process
What is the definition of Temporally Separated?
It is where distinct thermodynamic cycles, or specific stages within a single cycle, occur at different, non-overlapping points in time within a larger system or process
T or F: Brayton Cycle Steps are Temporally Separated
FALSE: They are Specially Separated
What is a Premixed Flame?
It is a type of fire where the fuel and oxidizer (like air) are thoroughly mixed together before they reach the flame front, allowing for rapid, uniform combustion that propagates through the entire mixture
(mnemonic - Think of Fuel Molecules as Gentlemen and the Oxidizer Molecules Ladies in a 1950's style debutant ball [in other words, only ladies can dance with gents... I am gay btw [not that it matters lol] so as heteronormative as this is, for the sake of clarity let's roll with the it :)]
In a Premixed Flame, all couples are paired up and ready to dance (dancing being the excited molecules at compression then ignition))
What is a Diffusion Flame?
a type of combustion where the fuel and oxidizer (like air) are not premixed and must mix at the flame front itself for burning to occur.
(mnemonic - Think of Fuel Molecules as Gentlemen and the Oxidizer Molecules Ladies in a 1950's style debutant ball [in other words, only ladies can dance with gents... I am gay btw [not that it matters lol] so as heteronormative as this is, for the sake of clarity let's roll with the it :)]
In a Diffusion Flame, only Ladies are in the chamber, they are then compressed and ready to dance, they just need a partner. Then the gents pour into the room, but the more gents enter, the more dancing pairs form, and as soon as they pair up, they start dancing (compression then ignition) so the other gents exerting the room have to squish past the already dancing couples to find their own pair. In other words, it's slower to burn in a Diffusion Flame than a Premixed Flame)
What does it mean to 'run Fuel Lean,' and how does it relate to a Diffusion Flame?
Of a Diffusion Flame, in the balanced stoichiometric chemical equation of Fuel + Oxidizer, in reality, the chemical reaction results in Fuel Left over after it has taken place. To reduce wasted Fuel, Diffusion Flames often 'run Fuel Lean' meaning they add an excess of the Oxidizer to the compression before squirting in the Fuel. This makes it so no Fuel is left over from the reaction, but you will instead be left with some unused oxidizer, which is more expendable in terms of cost. The down side to this is that is causes and Power Drop for the output of the cycle.
T or F: Gas Turbines use Fuel Lean, Lowering Combustion Temperatures (for material constraints) resulting in Low NOx
TRUE
What is the main cause for the Brayton Cycles Low Efficiency? What good, if any results from this?
Efficiency is generally Low due to inefficient Compression and Expansion, resulting in High Temperature Exhaust. BUT, this makes Brayton Cycles excellent for a Topping Cycle (Cogeneration) in a Combine Cycles (ex. a Power Plant)
T or F: Otto and Diesel Cycles have Temporally Separated Steps
TRUE
What kind of Flame/Charge does an Otto Cycle use?
Premixed Flame ; it requires auto-ignition, but the Power Increases
What kind of Flame/Charge does a Diesel Cycle use?
Diffusion Flame ; it runs Fuel lean, doesn't cause auto-ignition, but Power Drops; lower power density but higher efficiency!
What are the 4 steps in a 4 Stroke Engine?
1. INTAKE - The piston moves down, the intake valve opens, and a mixture of air and fuel is drawn into the cylinder
2. COMPRESSION - Both valves close, and the piston moves up, compressing the air-fuel mixture to increase its pressure and temperature.
3. POWER (or Combustion) STROKE - A spark plug ignites the compressed mixture, causing a controlled explosion that forces the piston powerfully downward, generating power.
4. EXHAUST - The exhaust valve opens, and the piston moves up again, pushing the burnt gases out of the cylinder.
What are the 2 steps in a 2 Stroke Engine?
1. COMPRESSION STROKE (or UP-STROKE) - The piston moves up, compressing the fuel/air/oil mix in the combustion chamber. Simultaneously, the upward movement creates a vacuum in the crankcase, pulling fresh fuel/air/oil through an intake port into the crankcase. Near the top, the spark plug ignites the compressed mixture, starting the power phase.
2. POWER STROKE (or DOWN-STROKE) - The ignited fuel expands, forcing the piston down (the power stroke).
As it moves down, the piston uncovers the exhaust port, letting burnt gases escape. It then uncovers the transfer port, allowing the fresh mix from the crankcase to rush into the cylinder, pushing out the remaining exhaust gases (scavenging) and filling the crankcase for the next cycle.
What are the 4 steps in a Wankel and Rotary Engine?
1. INTAKE - As the rotor turns, one of its faces uncovers the intake port, creating a vacuum that draws the fuel-air mixture into the expanding chamber.
2. COMPRESSION - The rotor continues rotating, reducing the volume of the chamber, which compresses the fuel-air mixture.
3. POWER (or Combustion) STROKE - Spark plugs ignite the compressed mixture, causing rapid expansion that forces the rotor to turn, generating power.
4. EXHAUST - The rotor's rotation moves the spent gases towards the exhaust port, which opens as the rotor uncovers it, expelling the exhaust gases.
What is so special about Otto Engine Spark Ignition (SI)?
It has a HIGH Power Density, and is the most common power plant in the US automotive industry
T or F: Otto and Diesel fall under the ASA for the purposed of this class
TRUE
~WHAT ARE 4 PROCESSES IN THE OTTO CYCLE?~
1 -> 2 : ISENTROPIC Compression
2 -> 3 : ISOCHORIC Heat Addition
3 -> 4 : ISENTROPIC Expansion
4 -> 1 : ISOCHORIC Heat Rejection
What is the Compression Ratio of the Otto and Diesel Cycles?
Otto : r = v_1,4 / v_3,2 = v_max / v_min
Diesel : v_1,4 / v_2
~THERMAL EFFICENCY EQUATION OF A OTTO CYCLE~
η = Ẇ_net / Q̇_in
ALT EQ
η = 1 - [1 / (r)^(k-1)] = 1 - [T_1 / T_2]
where
r = Compression Ratio = v_1,4 / v_3,2 = v_max / v_min
(T_2 / T_1) = (v_1 / v_2)^(k-1)
What is the key idea to keep in mind for the Otto and Diesel cycle calculations? (Hint: think of the iso- states of the cycles)
Use INTERNAL ENERGY (u) instead of enthalpy (h)
[ISOCHORIC = Constant Volume = Internal Energy (u)]
[ISOBAURIC = Constant Pressure = Enthalpy (h)]