Group 2- The Alkaline Earth Metals
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
What is the trend in atomic radius down group 2?
The atomic radius gets larger because extra electron shells are added as you go down the group.
What is the trend in first ionisation energy down group 2?
First ionisation energy decreases down the group
This is because each element down group 2 has an extra electron shell compared to the one above.
This means shielding increases and the distance between the outer electrons and the nucleus increases so there is less attraction between the outer electrons and the nucleus
This makes it easier to lose outer electrons
What is the trend in reactivity down group 2?
Reactivity increases down the group as it gets easier to lose electrons further down the group.
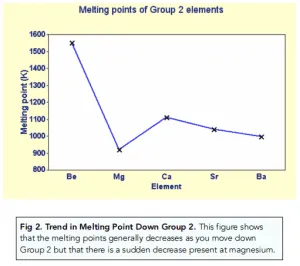
What is the trend in melting point down group 2?
Melting point generally decreases as metal ions get bigger down the group with the number of delocalised electrons staying the same and the charge
The larger the ionic radius, the further away the delocalised electrons are from the positive nuclei
So it takes less energy to break the bonds
However, there is an increase at Mg as the crystal structure changes