Final Exam - Developmental Bio
1/84
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
amniotes
A group of vertebrates that lay eggs on land or maintain embryos within the mother, including reptiles, birds, and mammals. Amniotes are characterized by having an amniotic egg, which provides a protective environment for the developing embryo.
amnion
A membrane that surrounds and protects the developing embryo within the amniotic egg, filled with amniotic fluid.
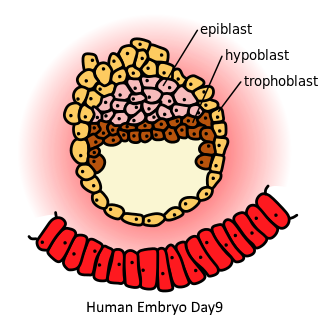
hypoblast
The layer of cells located beneath the epiblast in early embryonic development, contributing to the formation of extraembryonic structures and the definitive endoderm.
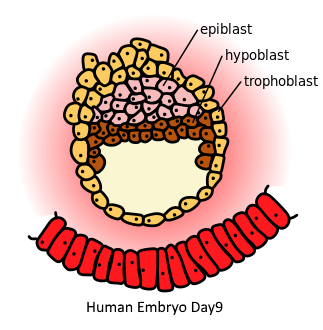
epiblast
single layer epithelium in amniote embryos that give rise to all tissues of the embryo proper through gastrulation
Koller’s sickle
crucial organizer in early bird development (gastrulation) where the primitive streak forms
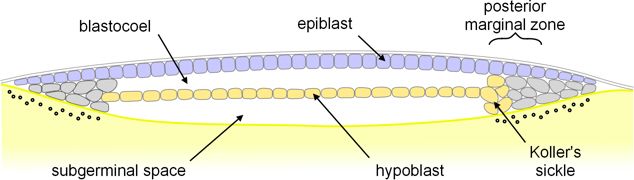
Primitive streak
The structure that forms during gastrulation in embryonic development, marking the beginning of the body plan and establishing the three germ layers.
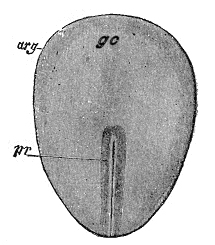
Hensen’s node
The organizing center at the anterior end of the primitive streak during gastrulation, essential for axial patterning and development of the notochord.
key organizer in early chick embryo
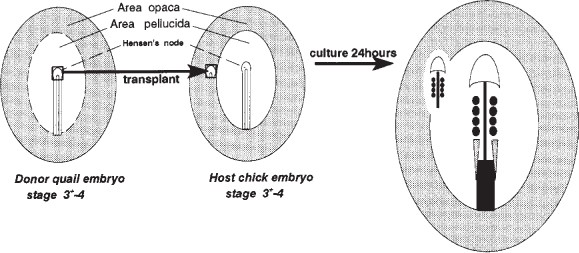
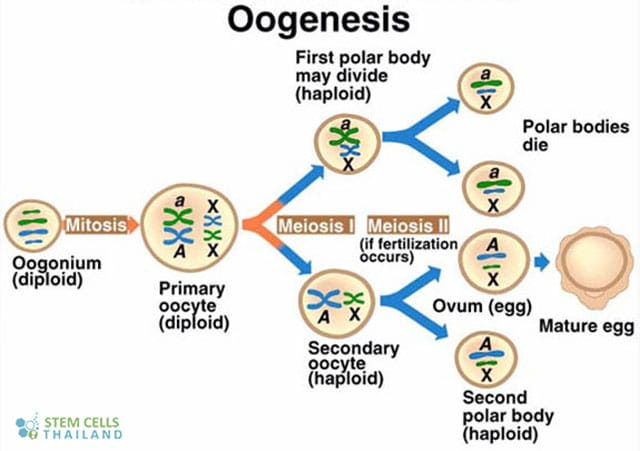
Polar bodies
small, haploid cells formed during oogenesis
zona pellucida
extracellular matrix surrounding eggs and early embryos
crucial role in initial gamete recognition
prevents polyspermy
protects embryo during its journey through the oviduct before implantation

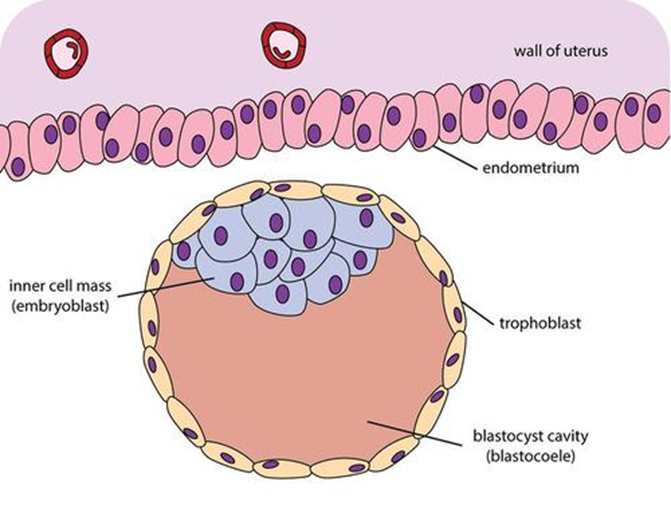
morula
solid ball of cells formed early in embryonic development, typically around 3-4 days after fertilization
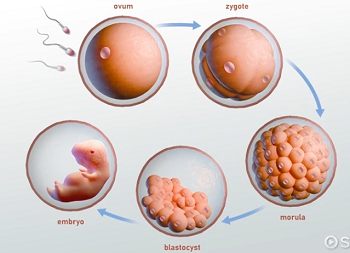
blastocyst
a hollow ball of cells formed from the morula, consisting of an inner cell mass that will develop into the embryo and an outer layer called the trophoblast that contributes to the placenta.
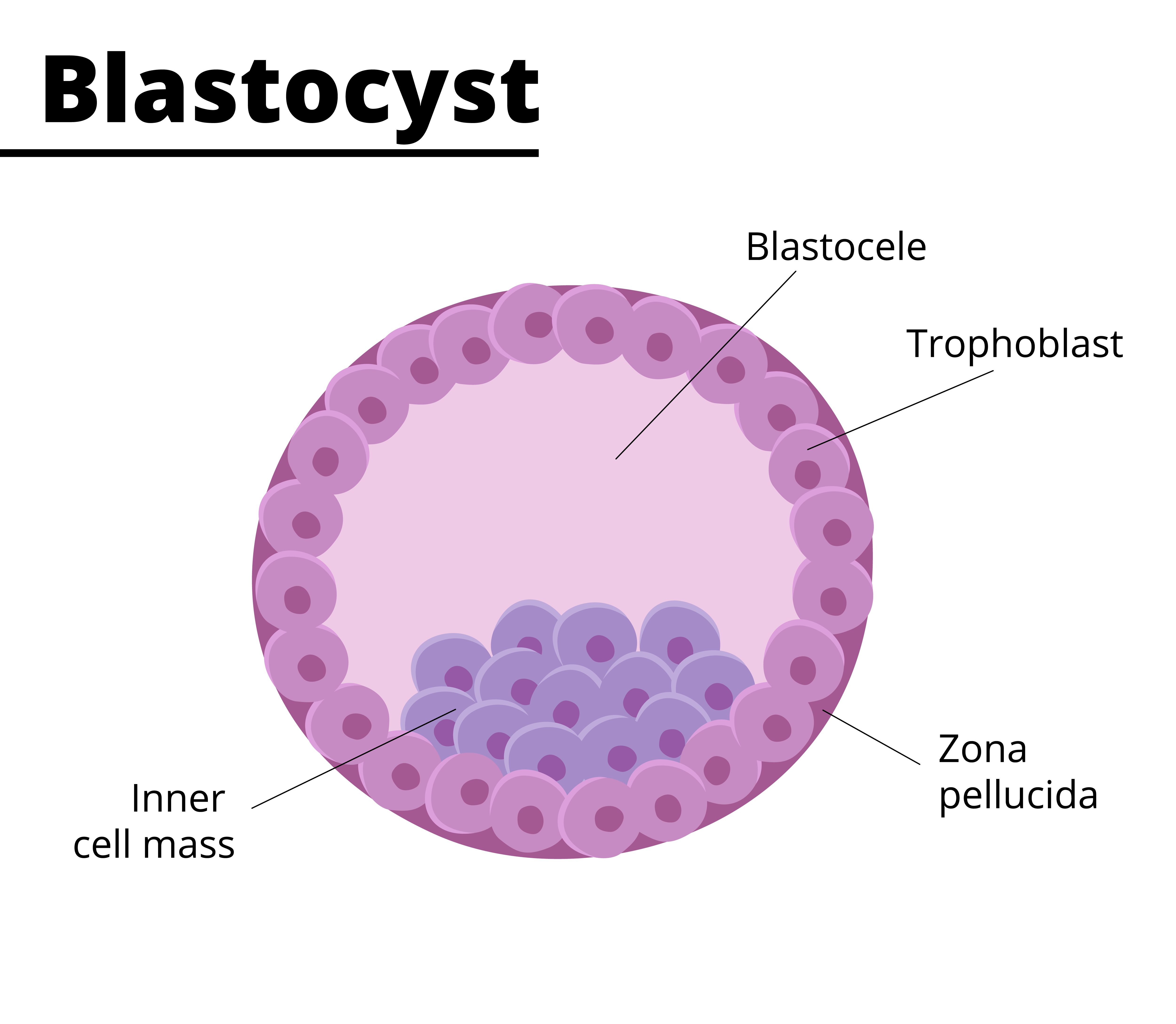
trophoblast
layer of tissue on the outside of a mammalian blastula, supplying the embryo with nourishment and forms major part of the placenta
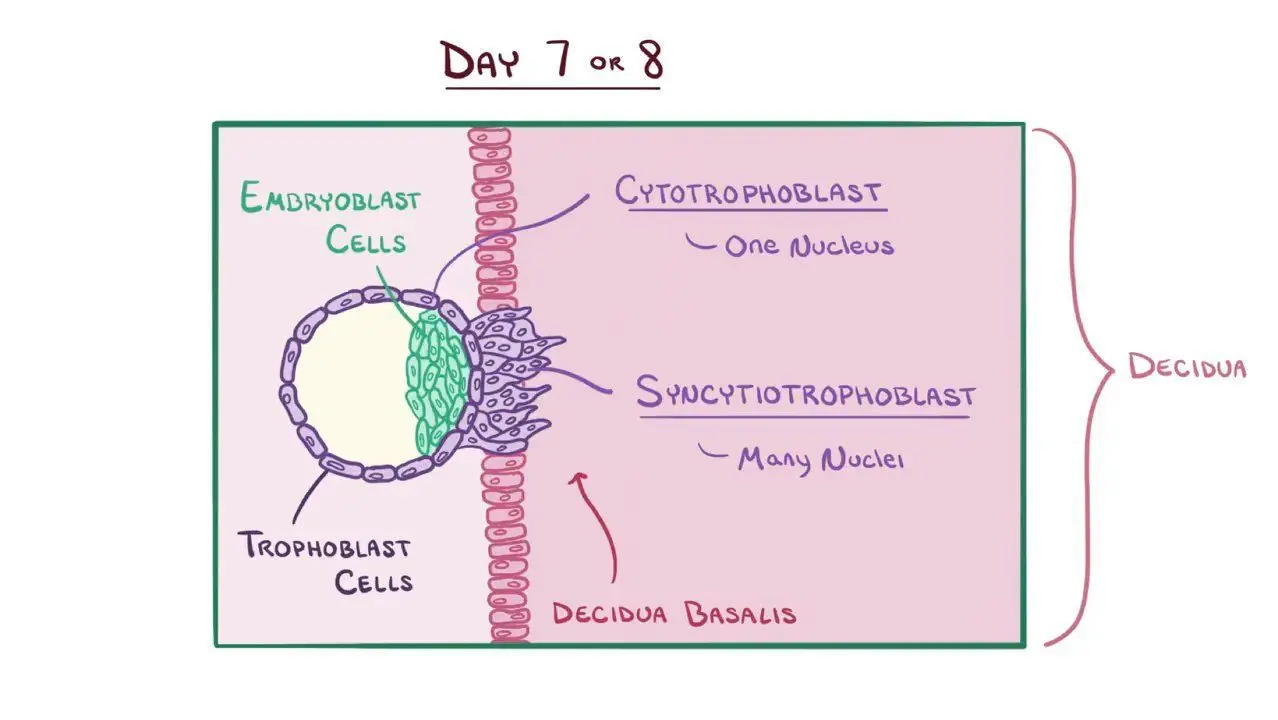
syncytiotrophoblast vs. cytotrophoblast
syncytiotrophoblast is the outer layer of the trophoblast that invades the uterine lining and facilitates implantation, while cytotrophoblast is the inner layer that provides mononucleated cells for growth and development of the placenta.
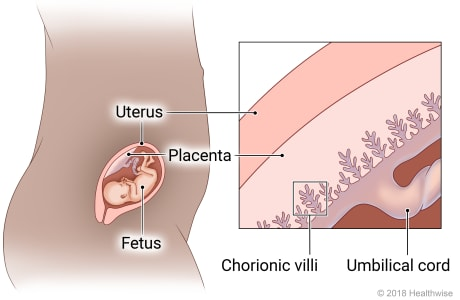
chorionic villi
finger-like projections forming the outer layer of the developing embryo and grows into the placenta
inner cell mass
embryoblast
cluster of cells within the blastocyst that develops into the embryo and some extra-embryonic tissues
morphogenesis
biological process that causes a cell, tissue, or organism to develop its shape
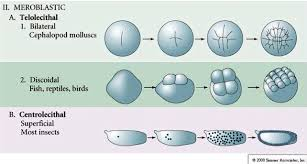
holeoblastic clevage
an entire zygote undergoes division, resulting in a complete cleavage of the egg cytoplasm into smaller cells (blastomeres)
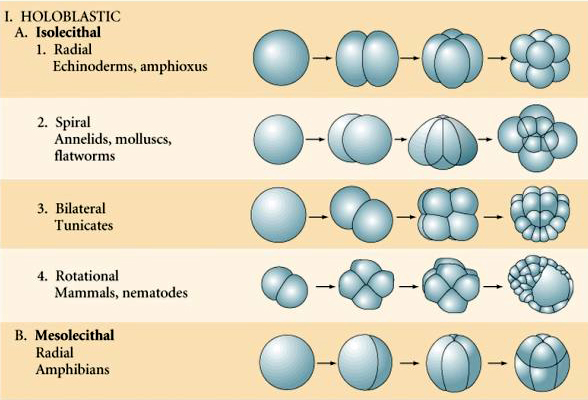
meroblastic clevage
incomplete or partial division of the egg cell
generally occurs in fertilized eggs that contain a greater quantity of yolk
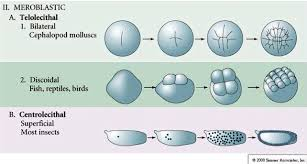
telolecithal vs. centrolecithal discoidal cleavage
Telolecithal cleavage involves uneven yolk distribution leading to a discoidal pattern of division, while centrolecithal cleavage occurs in eggs with yolk concentrated in the center, resulting in partial cleavage around the yolk.
Describe how the developing embryo is implanted in the endometrium.
sperm → egg = fertilization and blastocyst are formed, goes towards uterus to implant
after 5 days, blastocyst reaches uterus and created inner cell mass (which becomes embryo) and trophoblast (which will become placenta)
blastocyst creates enzymes to break down cells of endometrium to adhere to it
trophoblast invades deeper, creating syncytiotrophoblast and cytotrophoblast, and then eventually creating the placenta
placenta then creates blood vessels and secretes human chorionic gondadotropin hormone
How does a chorionic villi sample work?
prenatal test used to detect genetic abnormalities in developing fetus by taking a small sample of the chorionic villi from the placenta, which contains fetal DNA. The sample is then analyzed for chromosomal abnormalities or genetic disorders.
testing includes karyotyping and genetic testing
Outline how a chicken egg develops in the oviduct of the hen
yolk → formed in ovary
yolk released from ovary into oviduct
enters magnum where albumen begins to form
then enters isthmus, where inner and outer shell membranes form around the egg white
then the egg enters the uterus, where calcium carbonate is deposited to form the hard eggshell
before the egg is laid, a bloom is applied, then the egg is laid
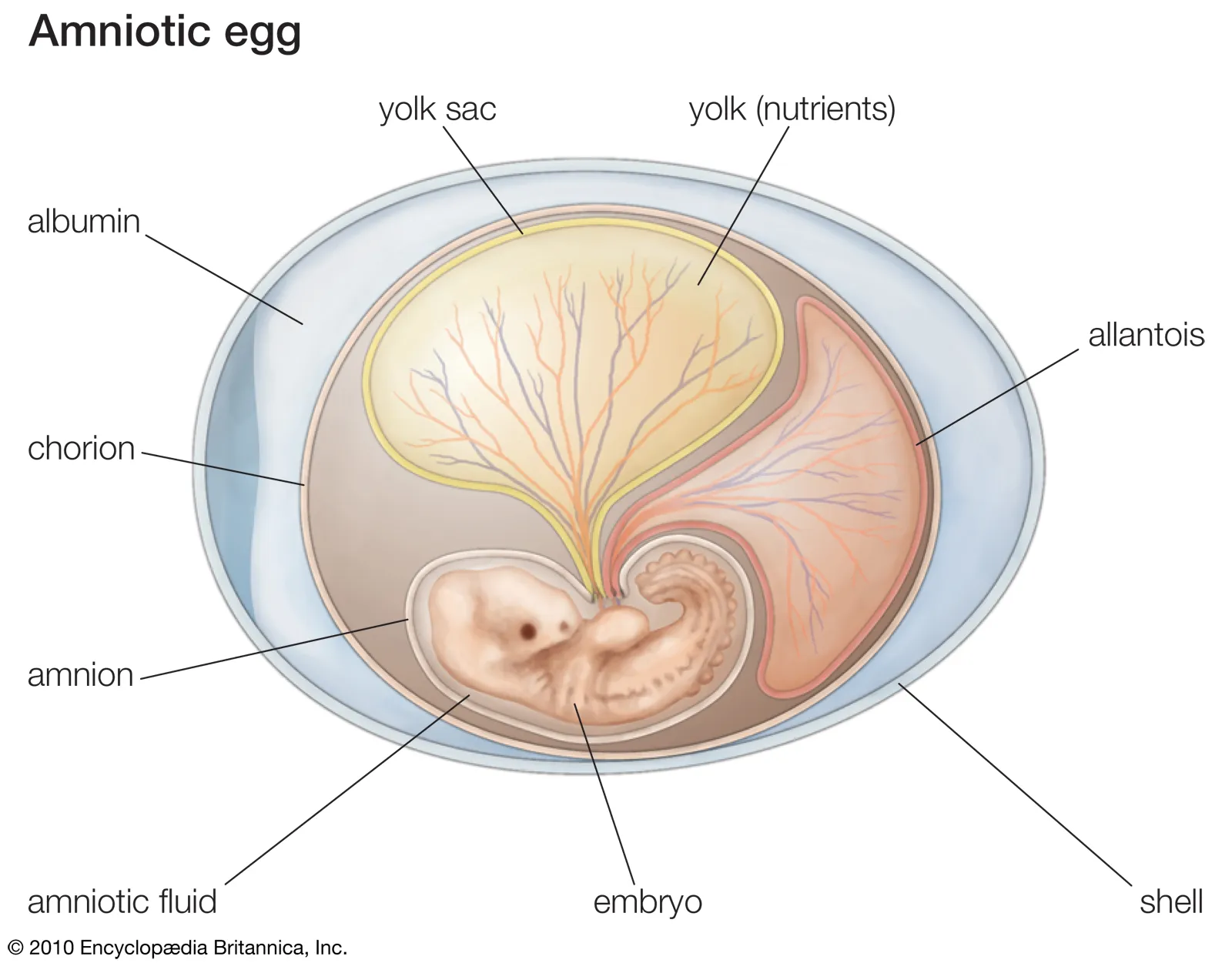
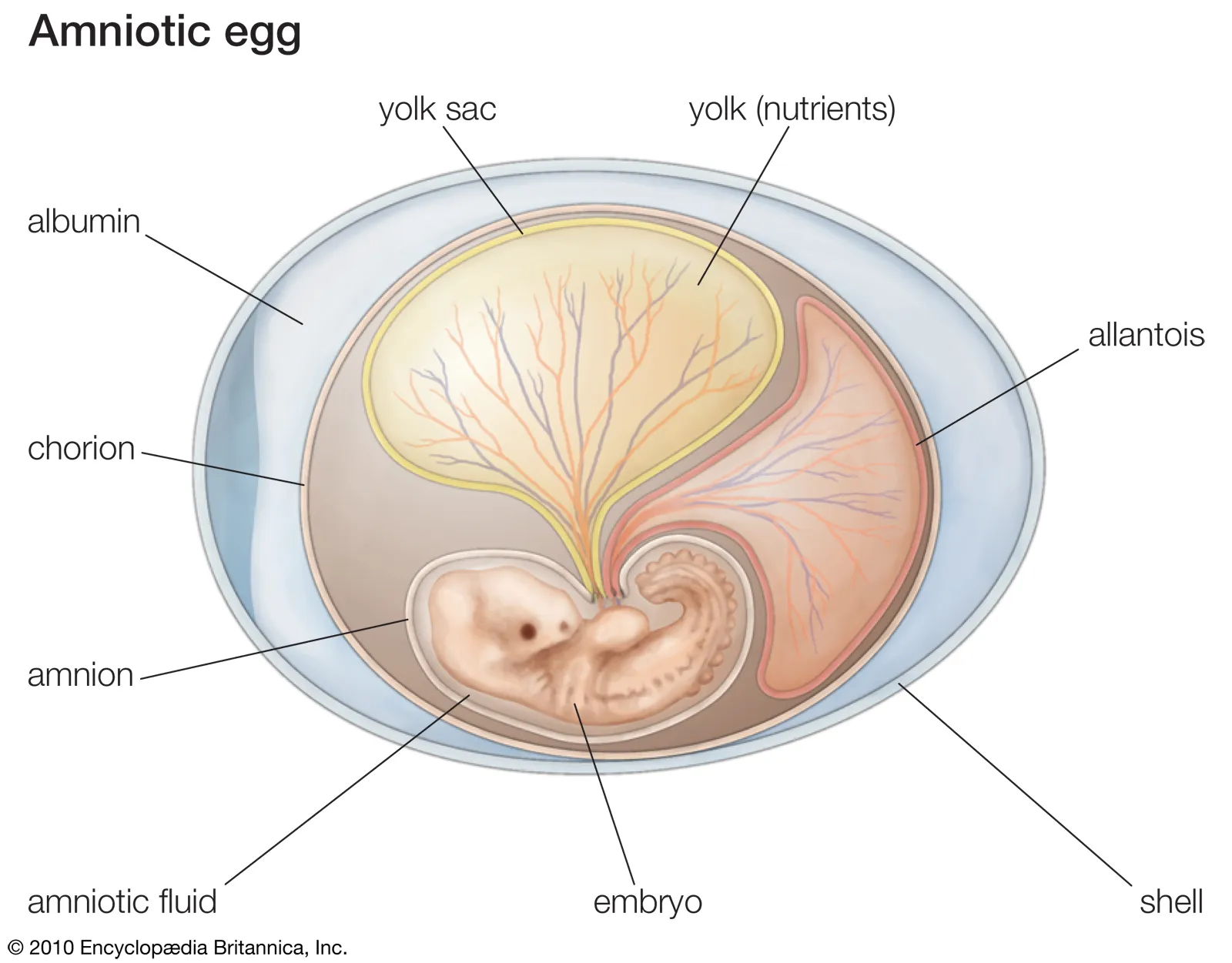
Identify the different extraembryonic membranes
amnion - protective membrane that holds amniotic fluid and cushions the embryo
chorion - outer membrane that assist with gas exchange and contributes to the placenta
allantois - involved in waste storage, gas exchange, and formation of umbilical cord
yolk sac - provides nutrients to the embryo early in development
nest cells
cluster of cells within organs or tissues
primordial germ cell
initial precursors of gametes during embryonic development
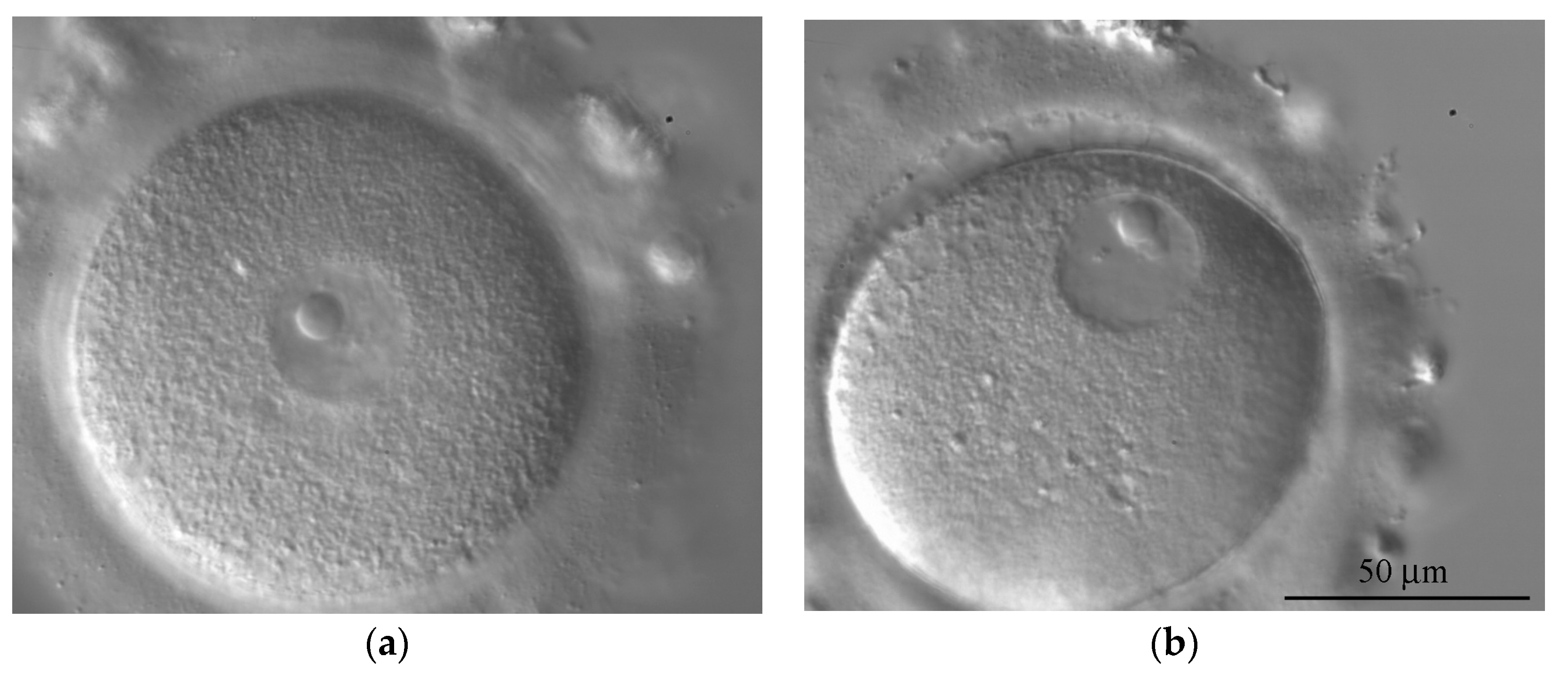
germinal vesicle
large, visible nucleus of a primary oocyte before it has completed its first meiotic division
vitelline envelope
protective, acellular layer surrounding the egg
follicle cells
specialized cells primarily found in the thyroid gland and in the ovaries
lampbrush chromosomes
large, looped structures formed by the uncoiling of chromatin in certain oocytes during diplotene stage of meiosis, allowing for active transcription.
Nieuwkoop center
organized gastrulation
a lot of vegetation region that had inducing factors to develop mesoderm
Explain how sperm entry sets up the anterior and posterior axis’s
sperm entry → cortical rotation, which re-localizes maternal determinants, and marks the posterior end of the embryo
cortical rotation → relocation of maternal determinants
proteins and RNA molecules that were initially distributed asymmetrically in the egg’s cytoplasm
formation of gradient of signaling molecules that defines anterior and posterior ends
once sperm enter eggs, markers and molecular signals initiate stabilization of embryo axes
posterior markers include Vg1 and beta-catenin
anterior markers are relatively unmarked
Identify and explain the genes involved in the dorsal/ventral axis formation.
Cortical rotation - triggered by sperm entry, plays crucial role in setting up axis by redistributing maternal determinants within the egg
Wnt/beta-catenin - central pathway involved in determination of the dorsal side of the embryo
TGF-beta - plays critical role in establishing the D/V axis
Nodal - secreted factor that is crucial for establishing the dorsal side of the embryo
BMP4 - key factor in formation of ventral region of the embryo
Chordin - antagonist that is highly expressed on the dorsal side of the embryo
Noggin - binds to BMPs and prevents ventralizing effects
Outline how the germinal vesicle becomes an egg.
Primary oocytes will become follicle cells
One of the 16 will become egg
Other eggs surround it
Focusing on one cell of nest and becomes an oocyte
Starts meiotic prophase
Long prophase (2 weeks)
Multiple nucleoli
Sub structure of nucleus
Ribosomes are assembled here
Make proteins
Lampbrush chromosomes
Bigger chromosome
Region of chromosome is amplified and makes a lot of RNA
Getting a lot of nutrients becoming the yolk
Follicle cells contribute to proteins becoming the yolk
Cell will become germinal vesicle
Protein being produced called vitellogenin
Made by mom’s live
Hormones from pituitary gland will produce progesterone
Gonadotropin
Signal for egg (diploid) to cause breakdown of the nucleus and go into meiosis 1
Produces polar body
Ready for meiosis 2 but it needs to fertilize in order to unfreeze
Now the frog can put the egg in the water
In order to release eggs, the male must “massage” the female frog
Amplexus
Male frog can release sperm right away
Laying eggs in the water = spawning
Cytostatic-factor
Can’t move the egg
X- Emi2
Rest the oocyte in meiosis 1
Mos
Allows entry of meiosis 2
Quick release of calcium
30 degree rotation once it enters meiosis 2
Helps set up dorsal and ventral sides
If it stayed in meiosis 1, it would be radial symmetry, but if it rotates, then it is bilateral symmetry (different types of cleavage)
Sets up anterior and posterior
hemimetabolous
having no pupal stage in the transition from larva to adult
holometabolous
insects that undergo complete metamorphosis
imago
adult fly
hypocotyl
part of the stem of an embryo plant beneath the stalks of the seed leaves and directly above the root
apomictic development
a form of asexual reproduction in plants where seeds are produced without fertilization.
molting
exoskeleton removed
corpora allata
endocrine glands near or attached to the brain
releases juvenile hormone
juvenile hormone (PTTH)
secreted from the brain
stimulates the prothoracic glands to produce ecdysone
ecdysone
hormone that triggers molting
imaginal disc
what takes larvae to imago
A tadpole is given Thyroxine. What will happen to the tadpole?
Stimulate pituitary gland
Stimulates TSH
Produce T4 and T3
Increase in thyroxine as they proceed to develop
We have thyroid T4 and T3 being released from the thyroid hormone
Stimulates hypothalamus
Once it reaches climax, then it is blocked and is no longer self regulating
Explain the hormonal control for metamorphosis in insects. Include the organ(s) responsible for the synthesis of the hormone(s).
Ecdysone - primary hormone responsible for triggering molting and initiation of metamorphosis by binding to ecdysone receptors in insect’s epidermis; they rise as insect approaches time to molt; also regulates pupation
Prothoracic gland and corpora allata are primary organs secreting this to the brain and other regulatory factors
Juvenile hormone - regulates larval stage and prevents insect from prematurely undergoing metamorphosis into its adult form; controls timing of when an insect will complete its final molt and transition from larval stage into pupal or adult stage
Explain the hormonal control for metamorphosis in frogs. Include the organ(s) responsible for the synthesis of the hormone(s).
Thyroid hormones, primarily thyroxine (T4), control metamorphosis in frogs by stimulating growth and transformation from tadpole to adult form. The thyroid gland is responsible for synthesizing these hormones, which trigger developmental changes.
ecdysone activates transcription of genes. List three of these genes.
BR-C - encodes for a family of zinc finger transcription factors
E74 - encodes for an ETS-domain transcription factor
E75 - encodes for a novel site-specific DNA binding protein
Be able to identify the imaginal disc and the body parts Drosophila become during pupation.
Describe how frog leg segmentation is formed, include genes/proteins
During frog development, leg segmentation is regulated by the expression of specific genes such as Hox genes, which provide positional information along the body axis. Proteins like FGF and Wnt are involved in the patterning and formation of the limb mesoderm, guiding the development of distinct leg segments.
Explain how the different organ systems change in the frog during metamorphosis.
Gills to lungs
Movement changes
Herbivore to carnivore
Gut is very lengthy, becomes more compact
Movement of the eyes changes
Vision gets developed
More terrestrial eye
Nitrogen excretion
Ammonia (gas) to urea (liquid)
Skin needs to go from thin to thick and have mucous glands
instar
developmental stage in arthropods that occurs between 2 successive molts
larva
active immature form of an insect
pupa
insect undergoes complete metamorphosis
ovariole
basic unit of an insect ovary
tubular structure where eggs develop
Drosophila has 2 ovaries
germarium
anterior or front portion of an ovariole where germ cells are produced
vitellarium
modified part of the ovary that flatworms and rotifers produce yolk-filled cells serving to nourish eggs
nurse cells
specialized cells that support the development of other cells, especially in the reproductive process
follicle cells
specialized cells found in the thyroid gland
pole cells
specialized cells in early insect and vertebrae embryos giving rise to germ cells
maternal vs. zygotic genes (examples and definitions)
maternal genes are genes whose produces are produced/deposited in the oocyte and are present in the fertilized egg/embryo before the embryo’s own genes start expressing. examples include mitochondrial DNA
zygotic genes are genes expressed in the early embryo, marking as transition from relying on maternal genetic information to the embryo’s own genome. examples include nanos and Bicoid.
gap genes (examples and definition)
gap genes are a group of segmented genes in early embryonic development that, when mutated, cause the loss of multiple contagious segments, creating gaps in the embryo’s body plan
examples include hunchback, knirps, and Krüppel
pair rule genes (examples and definitions)
the first genes to be expressed in periodic patterns in Drosophila embryo
examples include hairy, even-skipped, runt, and fushi-tarazu
segment polarity genes (examples and definition)
segment polarity genes are in Drosophila, and are crucial for defining/maintaining the internal structure of each segment in the developing embryo
examples include engrailed, wingless, hedgehog, and gooseberry
selector genes
class of genes (Hox usually) that regulate the expression of other genes, which ultimately determine the identity and development of body parts, tissues and cell types
they act as master regulators
Explain the progressional stages of Drosophila egg development starting in the germarium
starts in the germarium at the anterior end of the ovarian follicle → germline stem cells give rise to cystoblasts → oocyte selected and cyst is formed → axis specification (bicoid and nanos) → egg chamber forms → chorion formation and maturation of the egg → egg laid by the female
Identify what determines patterning in Drosophila-include all genes/proteins.
Maternal proteins: bicoid, nanos/hunchback - caudal
Important for setting up axis of developing embryo
Before egg
Zygote
Zygotic (gap): krüppel, hunchback, giant, abdomen, thorax
Pair rule genes: hairy, even skipped, rant
Segmentation of the developing fetus
7 segments
Segment polarity: engrailed, wingless
14 segments
Boundaries of segments (anterior and posterior)
Hox genes: antennapedia, bithorax, etc.
Identify the genes for anterior/posterior and dorsal/ventral axis
anterior/posterior:
bicoid
nanos
caudal
hunchback
kruppel
giant
knirps
even-skipped
fushi tarazu
runt
engrailed
wingless
hedgehog
antennapedia
dorsal/ventral:
gurken
toll receptor pathway
spätzle
dorsal protein
twist
snail
rhomboid
Toll receptor is used for dorsal-ventral axis. Outline the interactions of proteins/genes in the ultimate activation of twist and snail.
Fragment binds to TOLL - receptor
Toll receptors are in cancers and development
Activate Tube and Pelle to activate Cactus and phosphorylate cactus
Allows dorsal to be removed after phosphorylation
Cactus goes into nucleus
Twist and snail gets stimulated and represses Zen
Involved in expression of the mesoderm
Helps set up vaso dorsal segment
Moves to the side
Ultimately homeotic genes are expressed to give specific segment identity (head, thorax, abdomen). Discuss how they are regulated/expressed.
Homeotic genes, such as the Hox genes, are regulated by a combination of maternal and zygotic proteins that establish segmental boundaries and identities. They respond to gradients of transcription factors, including the anterior-posterior and dorsal-ventral gradients, influencing the activation or repression of specific genes to determine the fate of each segment in the developing embryo.
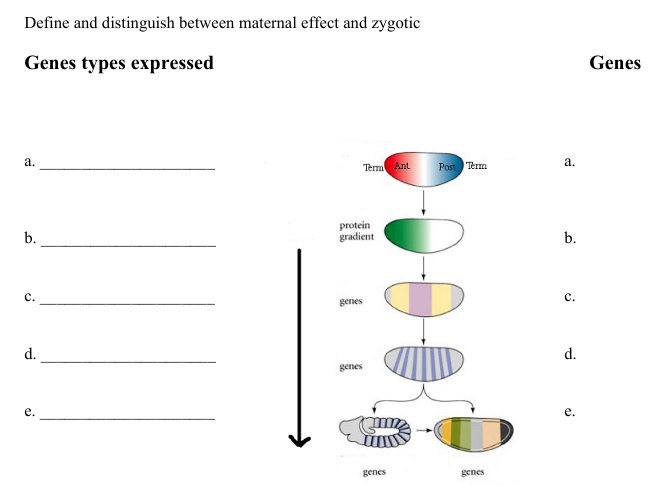
In the diagram below, put these genes Biocoid, Dorsal, wingless, kruppel, fushi tarazu, antennapedia , engrailed, nanos, even paired, hunchback (gene expressed) in order of expression for the development of the Drosophila larva. Label the diagram for where you would see pair-rule genes, homeotic genes, gap genes, segment-polarity genes and maternal effect genes (gene types)
a. bicoid
b. gap genes
hunchback
c. pair rule genes
even-skipped
fushi tarazu
d. segmentation genes
wingless
e. selector genes
abdominal-A
For the germline tissue below, what final tissue do they develop into…
endoderm
mesoderm
ectoderm
Endoderm develops into gastrointestinal tract and organs, mesoderm into muscles and circulatory system, ectoderm into skin and nervous system.
The eve strip results from a specific region of the even-skipped promoter. The promoter has binding sites for the activators (bicoid and hunchback) and repressors (giant, kruppel). The eve promoter DNA can drive expression in the strip pattern when fused to a reporter gene. Explain How and Why the pattern of the eve-reporter gene would change if the Kruppel binding sites in the promoter were removed.
Removing the Kruppel binding sites would allow the eve promoter to be less inhibited by Kruppel, potentially leading to increased expression of the eve gene in areas where it was previously repressed. This could result in a broader pattern of expression for the eve-reporter gene, as Kruppel acts as a repressor in specific regions, thus defining the spatial pattern of the eve stripe.
sHH, wnt and BMB proteins are involved in many areas of development, and we heard about them in multiple organ developments. What are they and what do they do?
sHH - sHH - Sonic hedgehog protein that regulates cell growth and differentiation during limb and brain development.
wnt - A group of signal proteins that play important roles in regulating embryonic development, cell proliferation, and tissue regeneration.
BMB - Bone morphogenetic proteins crucial for bone development and involved in the regulation of embryonic development.
telomere
protective DNA-protein structures found at the ends of chromosomes in eukaryotic cells
proteostasis
process by which cells maintain a healthy balance of proteins to ensure proper folding, functioning, and degradation of proteins.
proteosome
large, multiprotein complex responsible for degrading proteins within cells, primarily those marked for destruction by ubiquitin.
epigenetic
study of how the environment and other factors can influence gene expression without altering the underlying DNA sequence
mTOR
protein synthesis
inhibit and increased lifespan in mice
ROS
molecules produced during normal cellular metabolism, serving as important signaling molecules that regulate various cellular processes, like cell growth, differentiation, and immune responses
cellular senescence
stress/accumulation of damage over time causes cells to enter a state called cellular senescence
Also accumulate during the normal aging process in both humans and mice
Hayflick
parabiosis
in 2000 at Standford, scientists paired 2 mice, one old and one young. they peeled back their skin and stitched them together. they saw rejuvenation of muscles and livers in the older mice
other studies looked at the brain and mice improved on cognitive tasks and grew new neurons
deteriorated autophagy
Autophagy is recycling system in human cells
Body’s way of breaking down unwanted and diseased cell components and recycling them elsewhere
Evidence that stimulating autophagy increases lifespan and longevity in model organisms, highlighting importance of autophagy in the aging process
dysbiosis
Microbiome of older people is less complex and characterized by the presence of more pathogenic bacteria
Transferring gut microbiome from young to middle-aged fish was sufficient to increase their lifespa
Aging is an ongoing process. Summarize each of the twelve phenomena that contribute/causes aging.
Genomic instability
DNA damage and mutation
environmental factors
Radiation
External factors
Drugs
Can ionize
Can affect the lipid structure as well
Mitochondria
Reactive oxygen species
Errors in DNA replication
Antitumor drugs
UV-light chemicals
Hydrolysis
Replication errors
Telomere degradation
Get shorter as we age
Cancer cells have longer telomeres
Stem cell like
Doesn’t contain any genes
Starts to get damaged as DNA loses telomeric sequences
Loss of proteostasis
Accumulation of damaged and non-functional proteins
Misfolded proteins can clump together to form aggregates
Alzheimers and parkinsons
Improved protein turnover through activation of the proteasome or autophagy
Longer lifespan
Epigenetic changes
Changes in the modification of histones
Shown to affect lifespan of yeast, worms, and flies, suggesting that that the epigenome may not serve as a biomarker but also play a causal role in the aging process
DNA modification
Non-coding RNA
Imprinting
Histone modification
Impaired perception of nutrients
Dietary restrictions (DR)
Insulin and mTOR pathway form a central nutrient-sensing network within the cell, which has also been linked to the beneficial effects of DR
Mitochondria dysfunction
Main source of reactive oxygen species
Produced as a byproduct of mitochondrial respiration
Increased levels of ROS may even be beneficial, activating cellular defense and repair mechanisms
Cellular senescence
stress/accumulation of damage over time causes cells to enter a state called cellular senescence
Also accumulate during the normal aging process in both humans and mice
Hayflick
Exhaustion of Stem cells
Injecting blood plasma from young mice to old mice improves stem cell function in the old animals
Altered intercellular communication
Hormone, cytokines, metabolic products
Prabiosis
in 2000 at Standford, scientists paired 2 mice, one old and one young. they peeled back their skin and stitched them together. they saw rejuvenation of muscles and livers in the older mice
Deteriorated autophagy
Autophagy is recycling system in human cells
Body’s way of breaking down unwanted and diseased cell components and recycling them elsewhere
Evidence that stimulating autophagy increases lifespan and longevity in model organisms, highlighting importance of autophagy in the aging process
Chronic inflammation
Often occurs in aging tissues in low levels
Causes tissue damage and being implicated in the development of age-related diseases such as obesity and type 2 diabetes
Targeting inflammatory pathways in mice has been shown to rejuvenate tissues and improve lifespan
Imbalance of intestinal floral
Dysbiosis
Microbiome of older people is less complex and characterized by the presence of more pathogenic bacteria
Transferring gut microbiome from young to middle-aged fish was sufficient to increase their lifespan
Some older men take testosterone. What are the benefits of taking testosterone to combat aging?
improvements in sexual function, better mood overall, better muscle contractions, better fat distribution, higher sperm production