Energetics 2- IB CHEM Exam Review
0.0(0)Studied by 20 people
Card Sorting
1/6
Earn XP
Description and Tags
Last updated 7:08 PM on 4/18/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
How can you calculate the heat gained or lost by a substance?
The formula for calculating the heat gained or lost by a substance is Q = m × c × ΔT, where Q is the heat, m is the mass of the substance, c is its specific heat capacity, and ΔT is the change in its temperature.
2
New cards
What is specific heat capacity and what is the specific heat capacity of water?
\- Specific heat capacity refers to the “energy required to raise the temperature of 1 gram of a substance by 1 degree Celsius.
\- The unit for specific heat capacity is: __**J/g°C**__ (joules/(grams) x (degrees of temperature))
\- Every substance has a unique SHC.
\- The unit for specific heat capacity is: __**J/g°C**__ (joules/(grams) x (degrees of temperature))
\- Every substance has a unique SHC.
3
New cards
What is calorimetry and what law of nature governs it?
\- Calorimetry is the change in heat associated with a chemical process
\- It is governed by the First Law of Thermodynamics which states that energy cannot be created or destroyed, only altered in form and transferred.
\- It is governed by the First Law of Thermodynamics which states that energy cannot be created or destroyed, only altered in form and transferred.
4
New cards
How can calorimetry be used to calculate the ∆H of a reaction (both for combustion and in solution)?
Calorimetry can be used to calculate the ∆H of a reaction using a calorimeter. A calorimeter is an extremely insulated device which means that any temperature change is due to heat gained or lost during the reaction. Calorimeters are used to find the change in the value of heat (q)
5
New cards
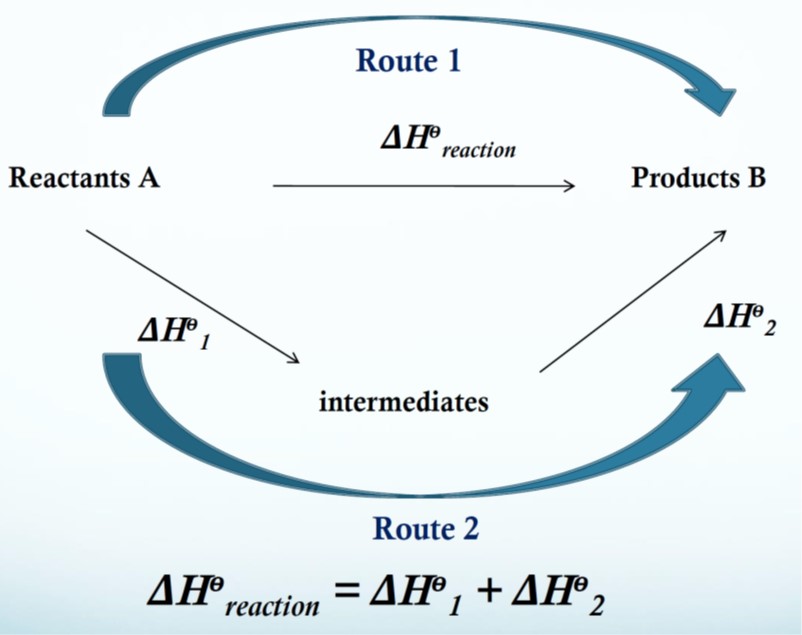
What is an energy cycle and what does one look like? (picture)
An energy cycle is a diagram that shows how three or more equations are interconvertible. They are used to find unknown energy changes.
6
New cards
What is Hess’s Law?
Hess's Law states that the total enthalpy change for a chemical reaction is independent of the route taken. In other words, the change in enthalpy of a reaction is the same whether it occurs in one step or in several steps. This law is based on the principle of conservation of energy.
7
New cards
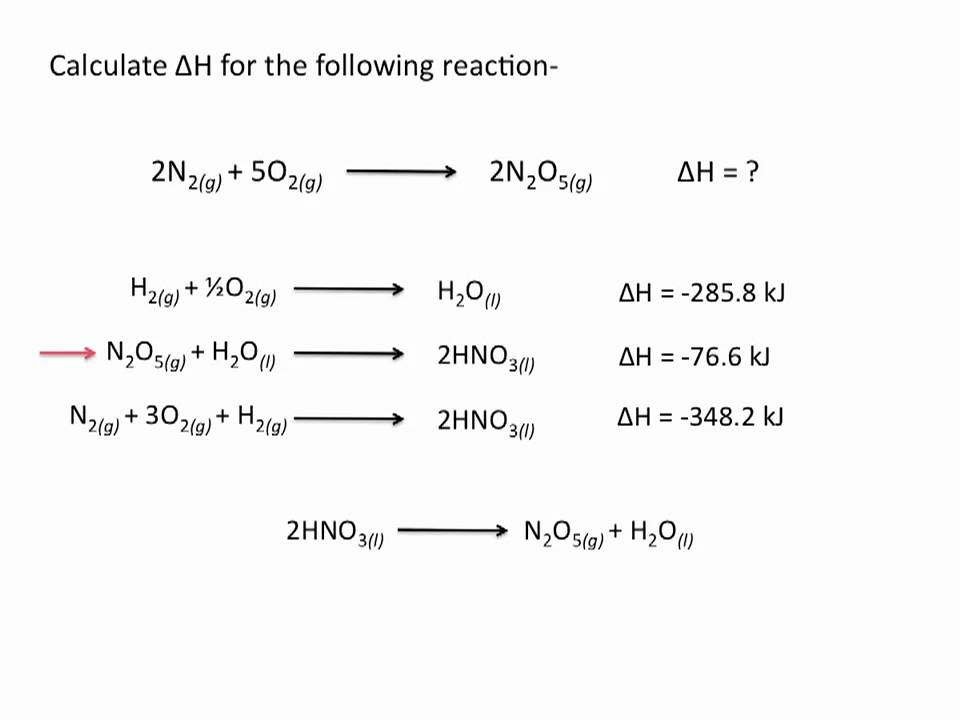
How can Hess’s Law be used to find the ∆H of a reaction both experimentally and theoretically? (example problems)
To find the enthalpy change (∆H) of a reaction using Hess's Law, one can measure the ∆H of multiple reactions that add up to the desired reaction.
\*Link to more practice problems: https://general.chemistrysteps.com/hesss-law-practice-problems/
\*Link to more practice problems: https://general.chemistrysteps.com/hesss-law-practice-problems/