Molecular Geometry and VSEPR
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Ideal Geometry
The central atom has no lone electron pairs.
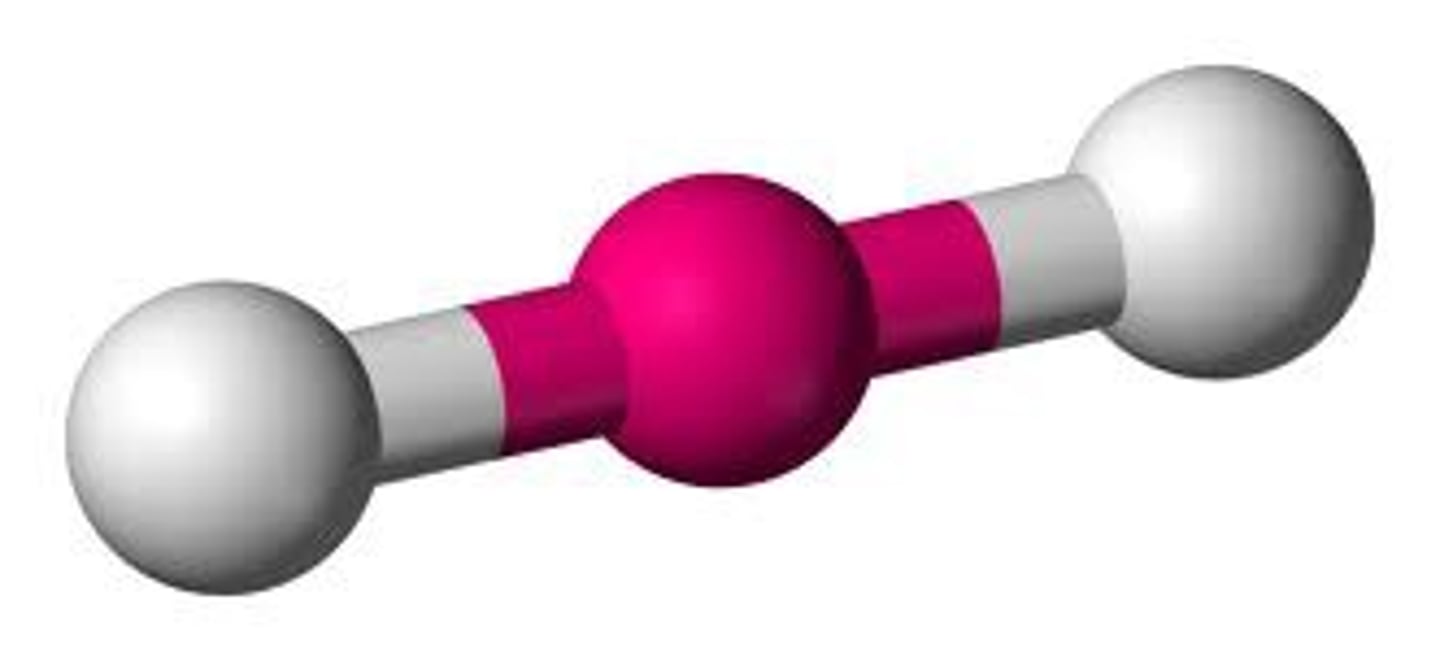
AX₂
Linear, bond angle 180°
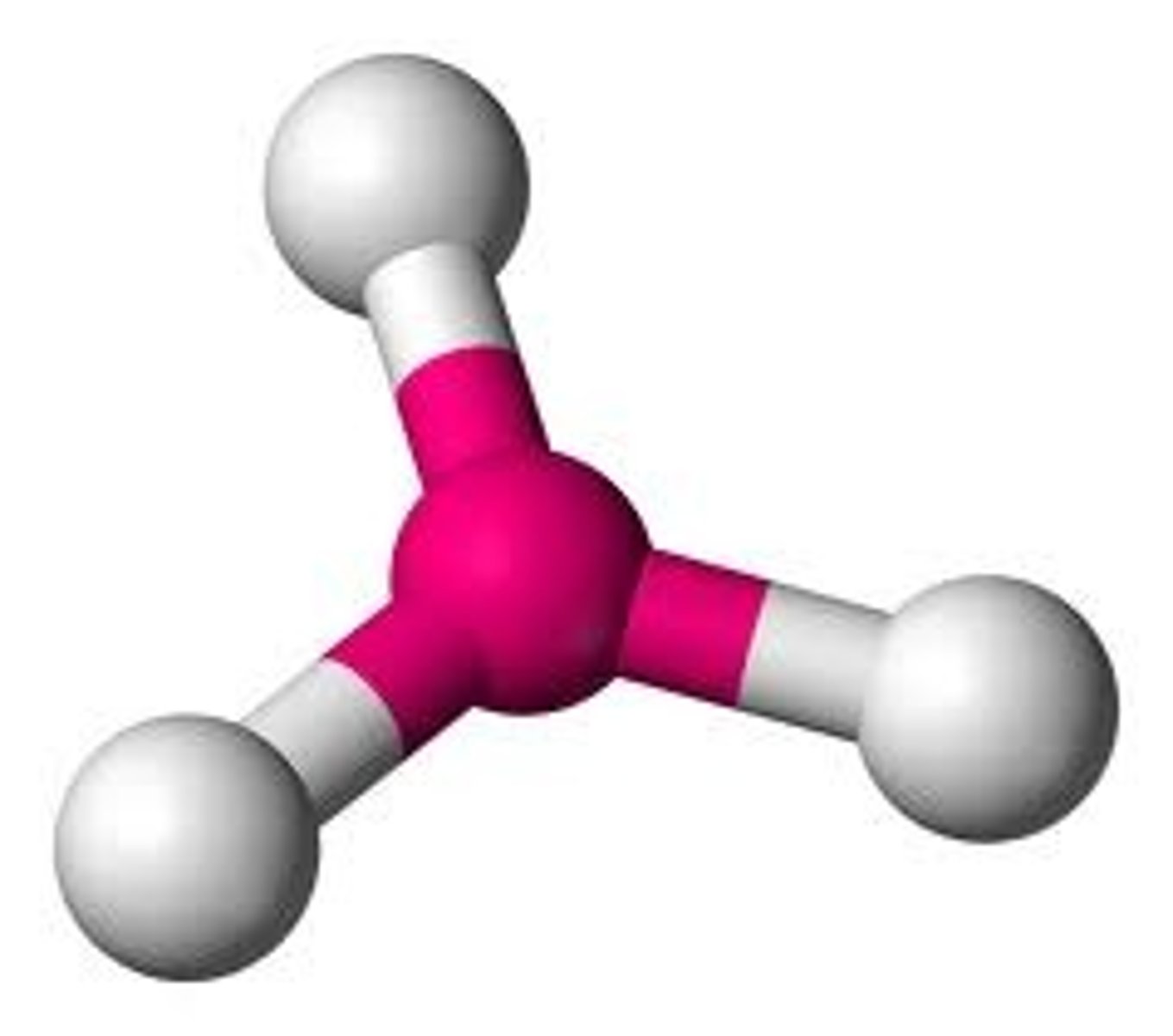
AX₃
Trigonal planar, bond angle 120°
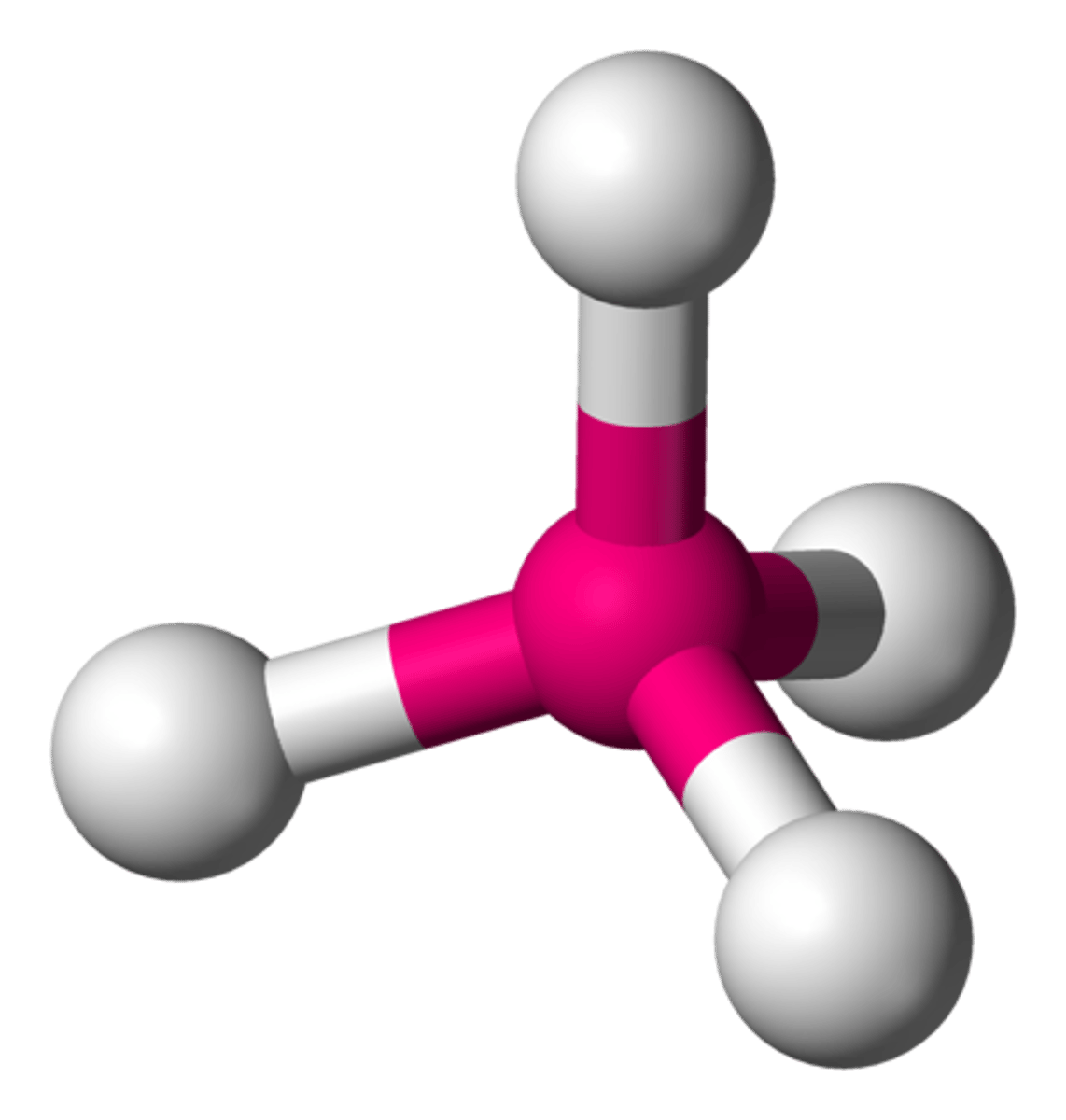
AX₄
Tetrahedron, bond angle 109.5°
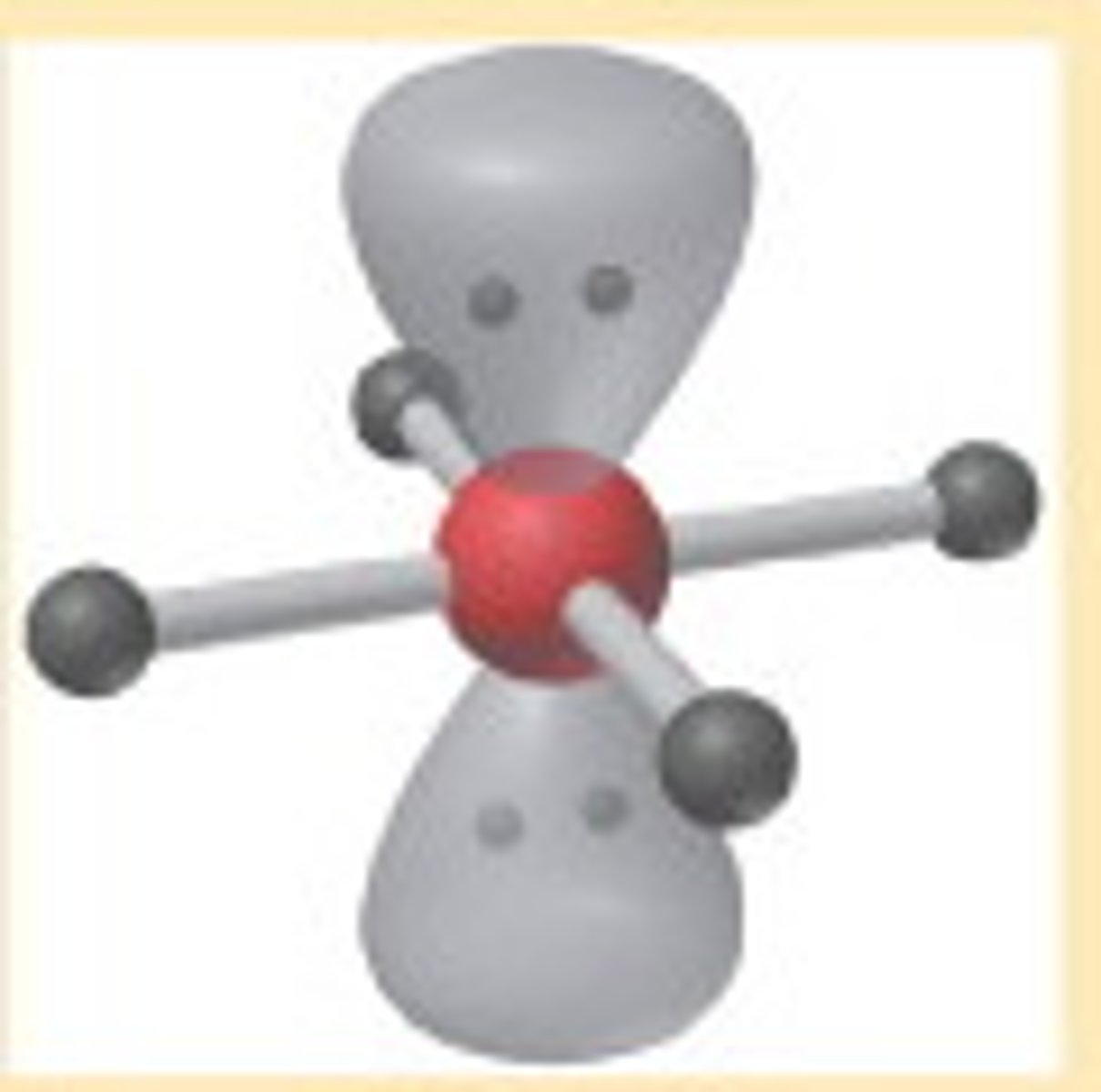
AX₅
Trigonal Bipyramid, bond angle 90°, 120°
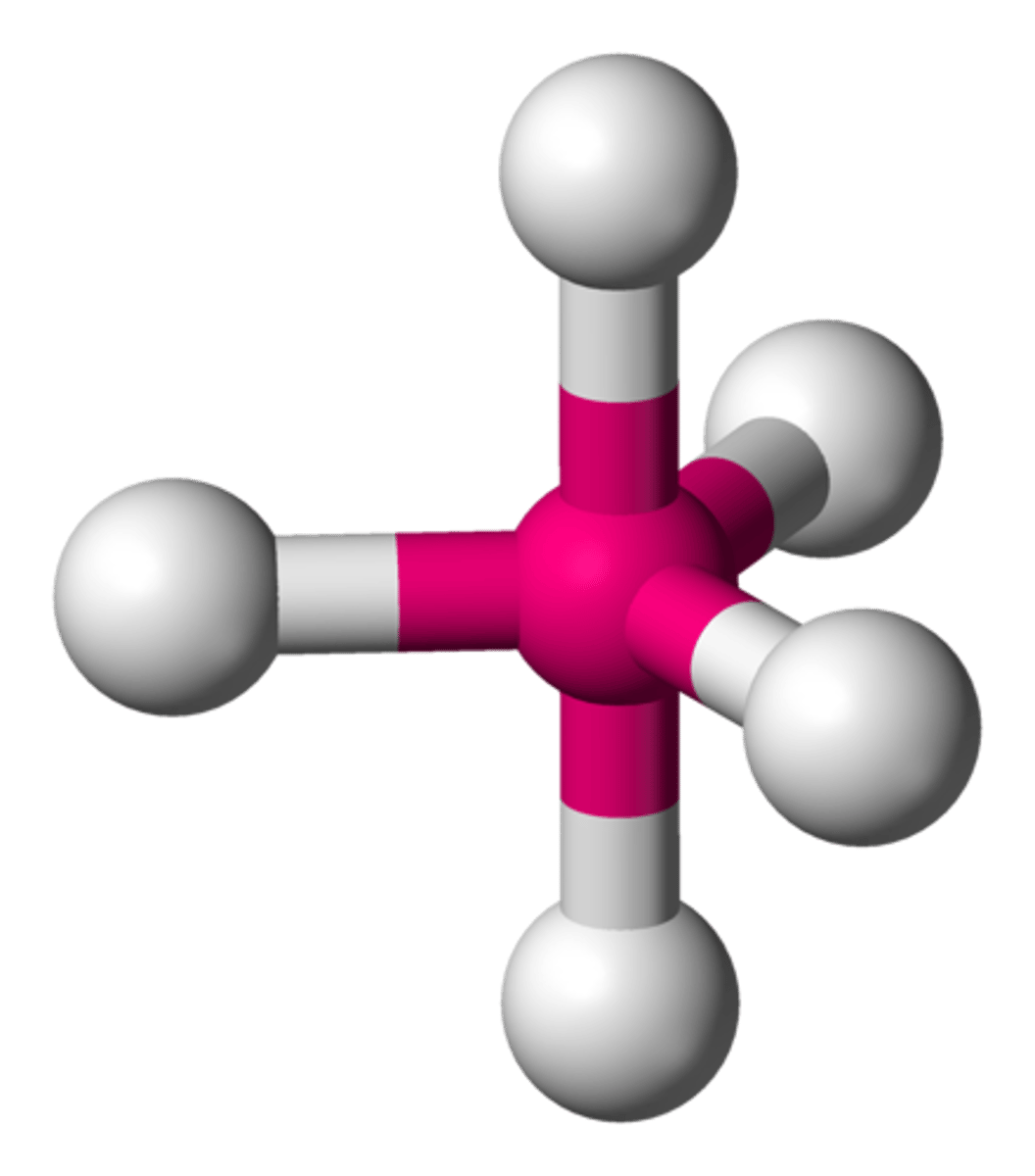
AX₆
Octahedron, bond angle 90°
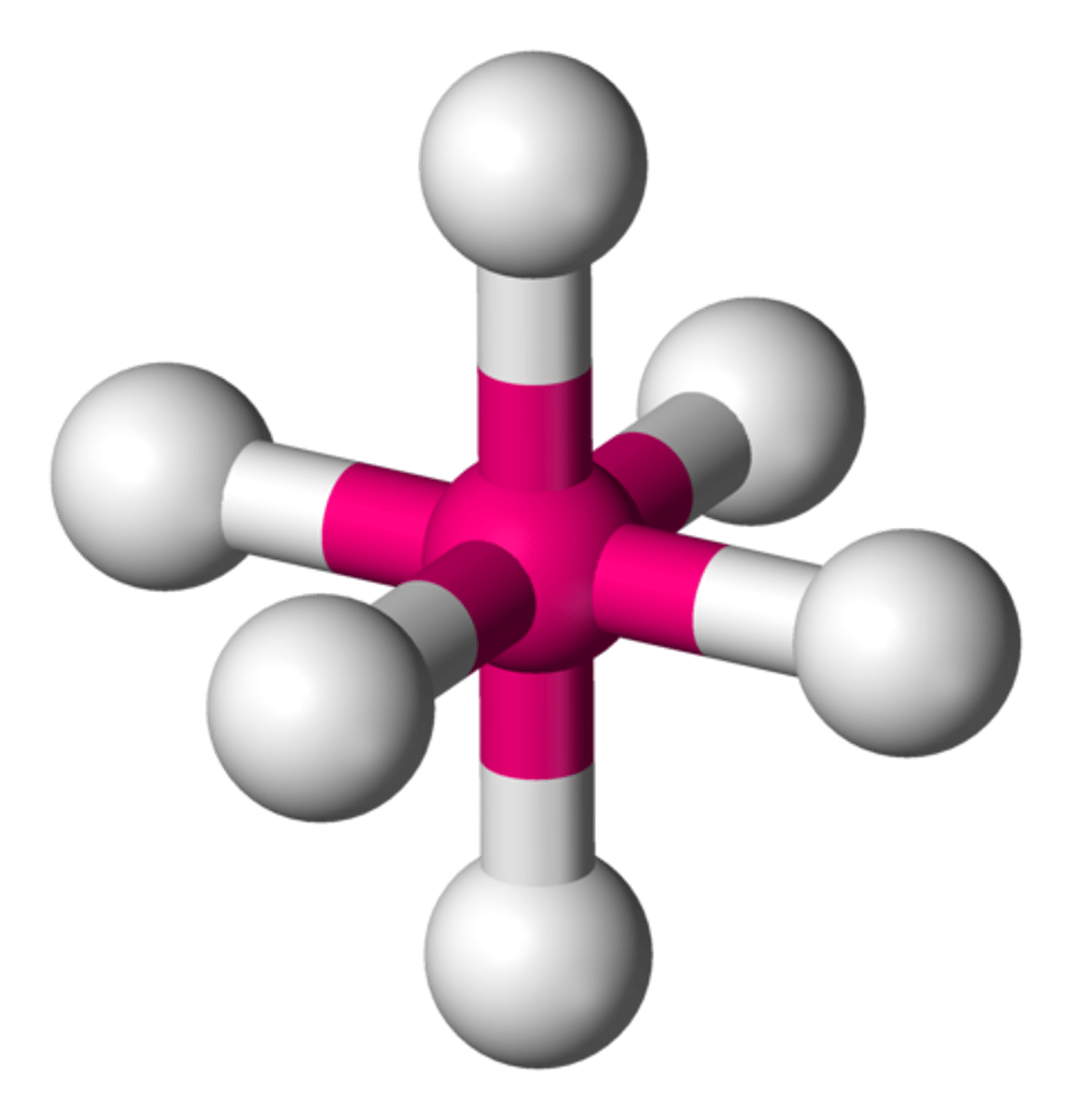
VSEPR Theory
Valence Shell Electron Pair Repulsion Theory:
-Double or single bonds can be treated like single bonds.
-If a molecule has resonance structures, VSEPR can be applied to any one of them.
-Lone pairs of electrons repel atoms more than bonding pairs do. Therefore, lone pairs take up more space than atoms.
AX₃E
Trigonal Pyramid geometry, tetrahedral arrangement of electron pairs, bond angle 107.3°
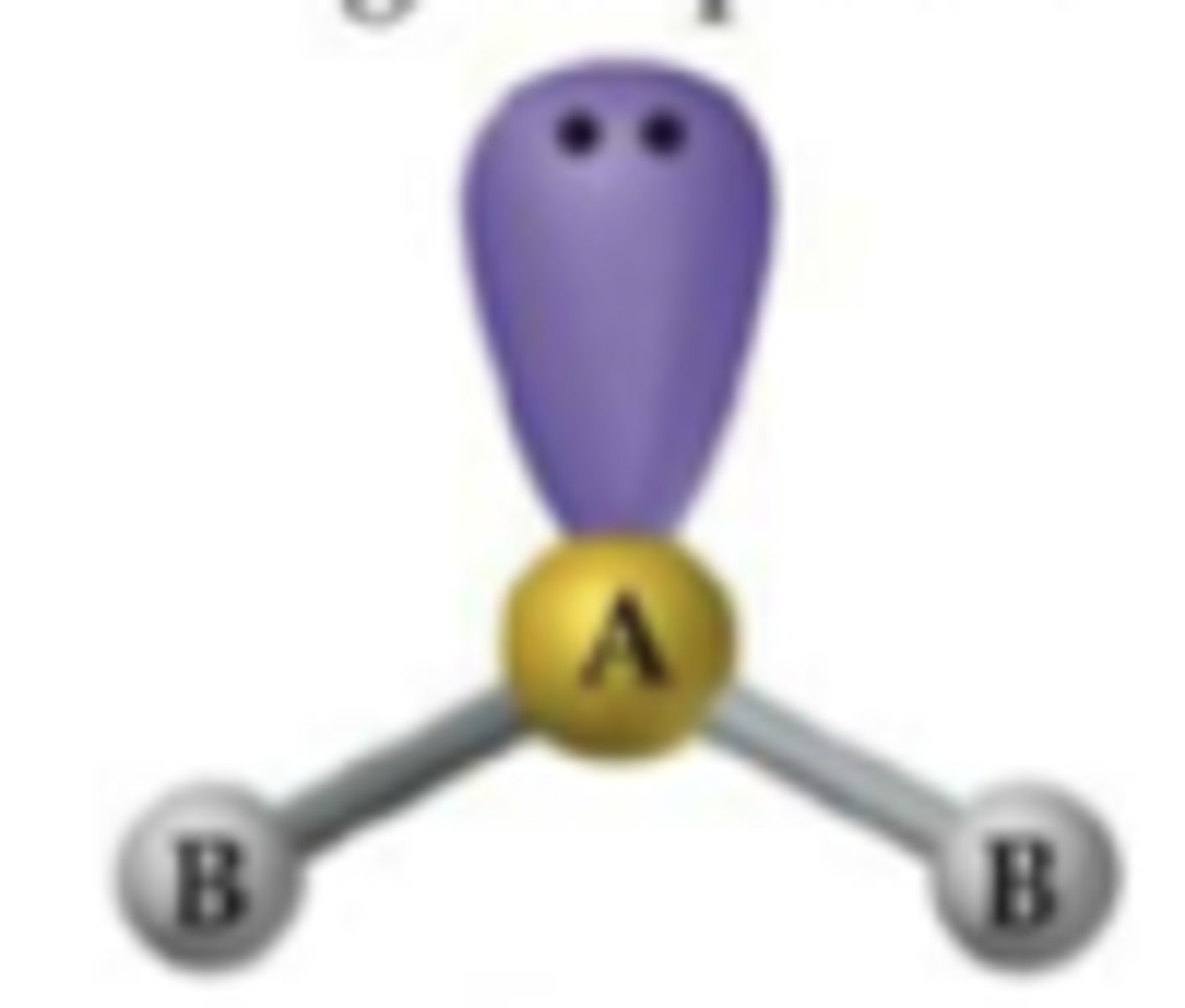
AXE₃
Linear, bond angle 180°
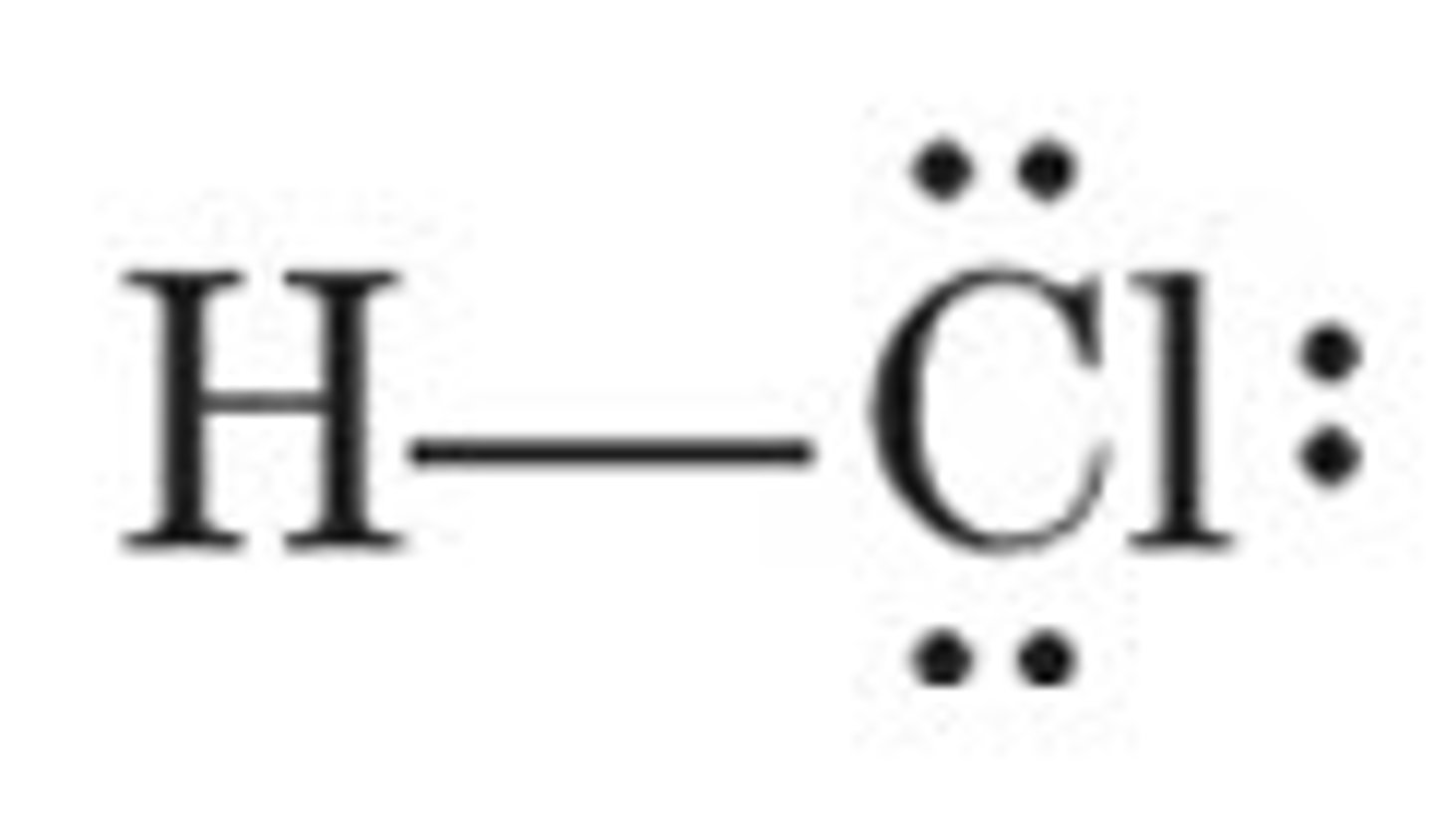
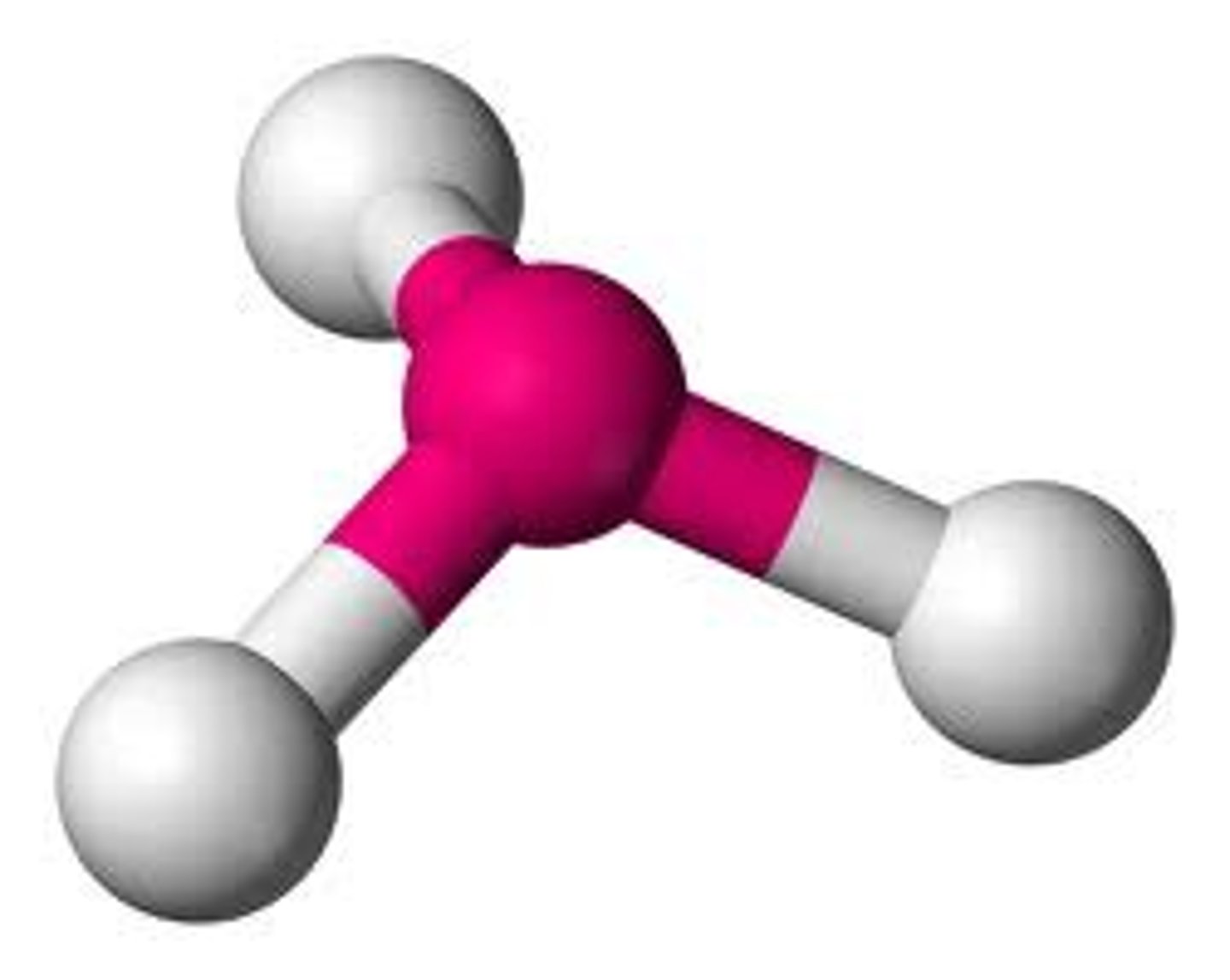
AX₂E₂
Bent geometry, tetrahedral arrangement of electron pairs, bond angle 104.5°
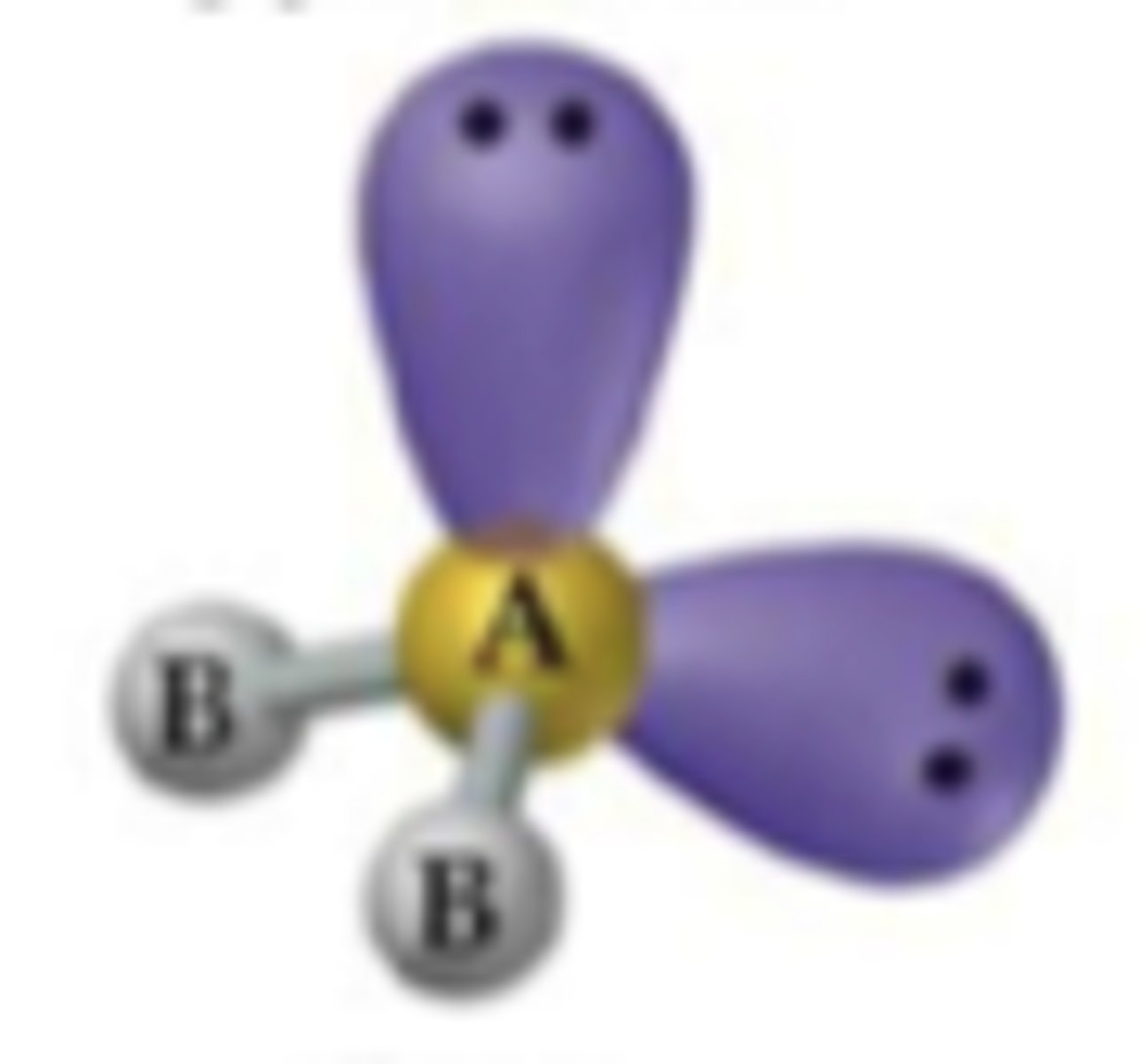
AX₂E
Bent geometry, trigonal planar electron pair configuration.
AX₄E
Seesaw/distorted tetrahedron geometry, trigonal bipyramidal electron pair configuration.
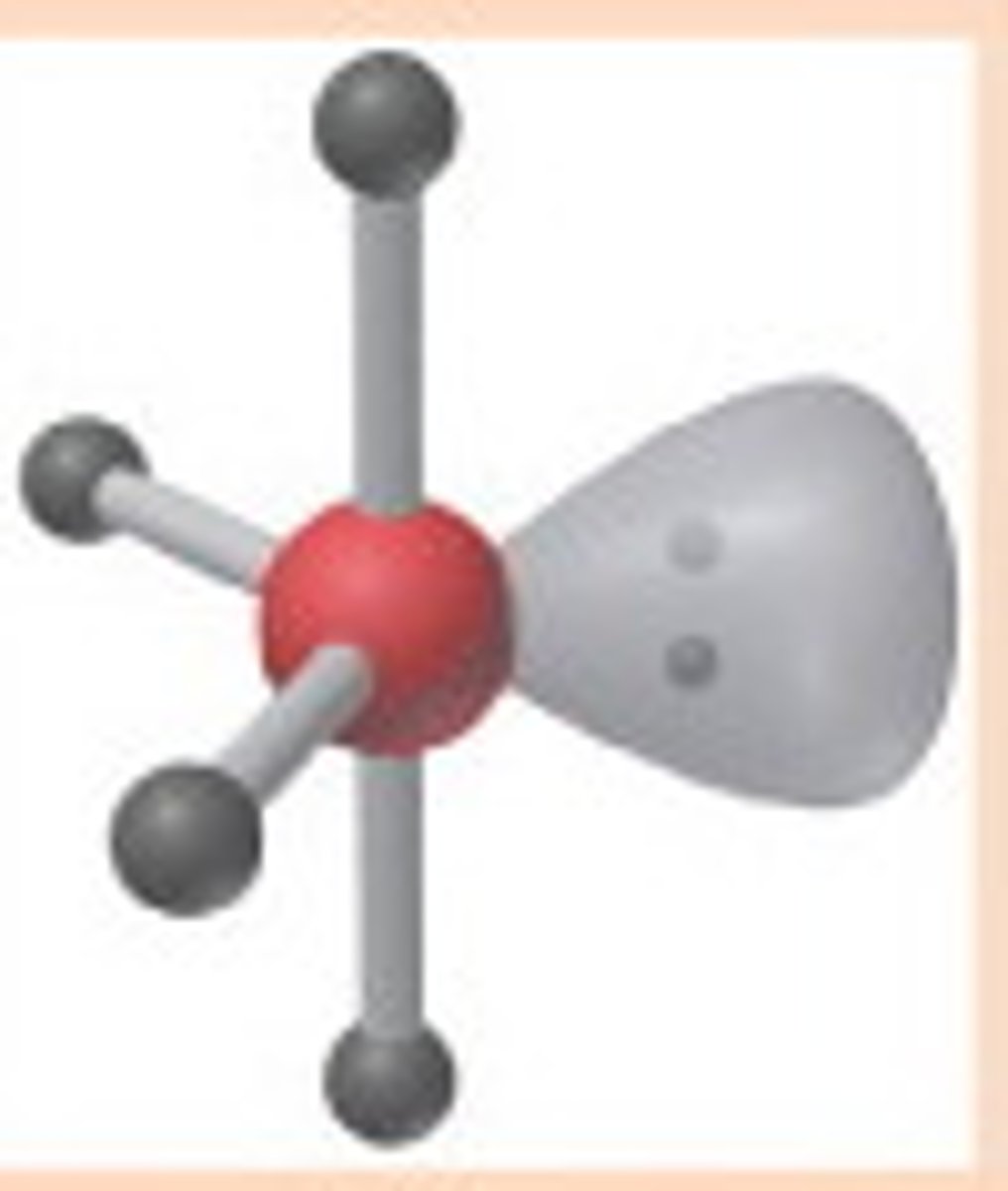
AX₃E₂
T-shaped geometry, trigonal bipyramidal electron pair configuration.
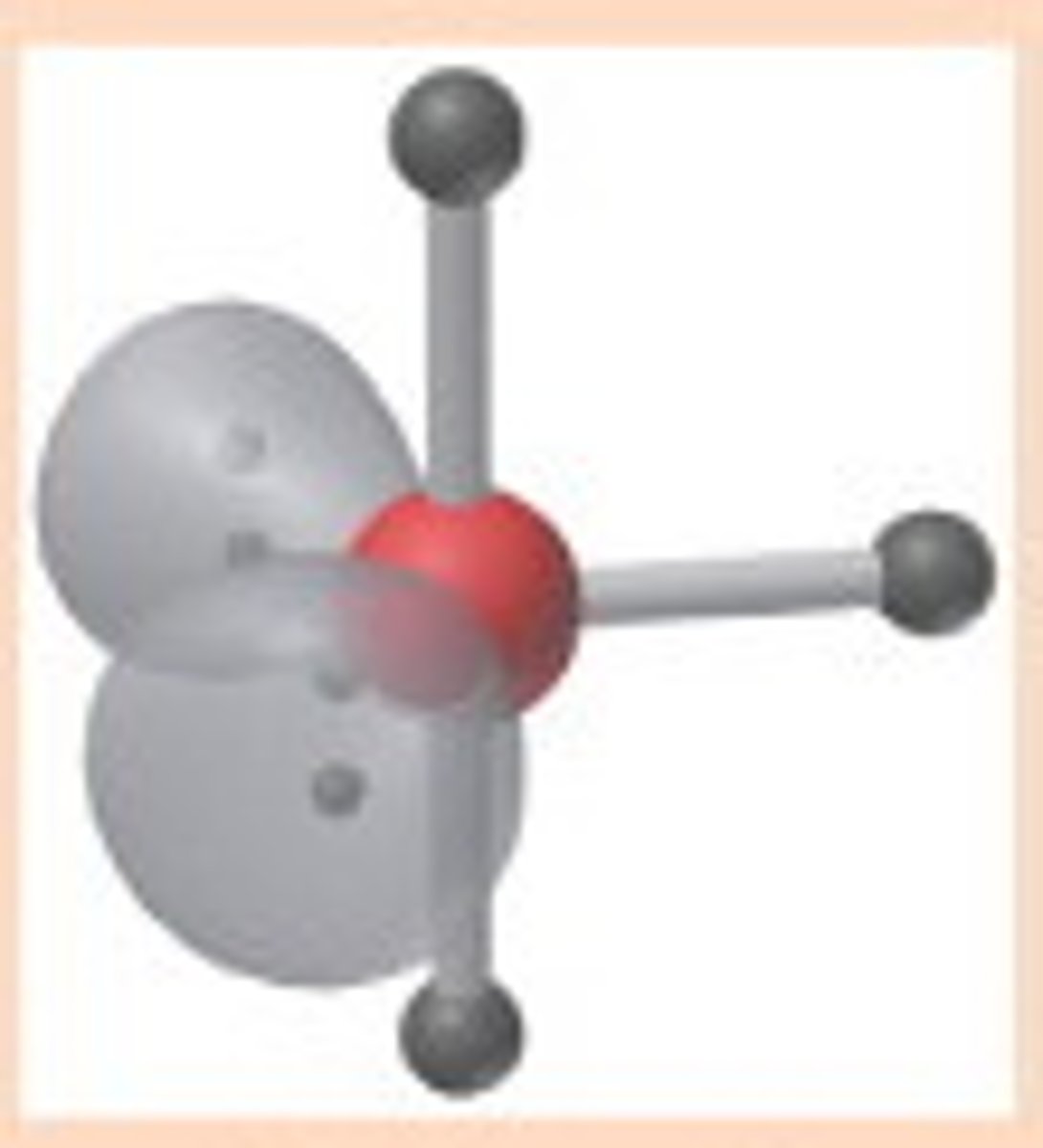
AX₂E₃
Linear geometry, trigonal bipyramidal electron pair configuration.
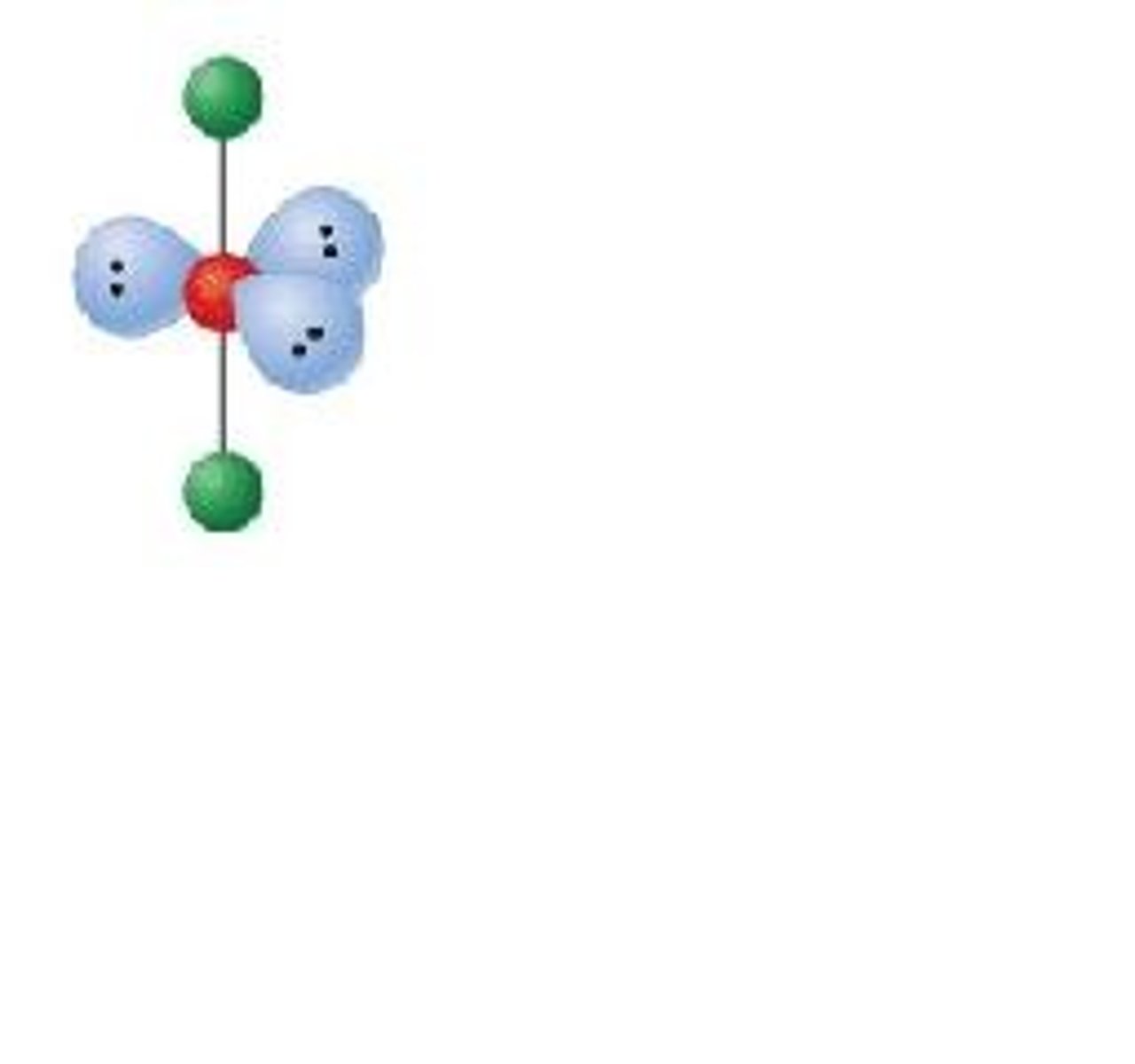
AX₅E
Square pyramidal geometry, octahedral electron pair configuration.
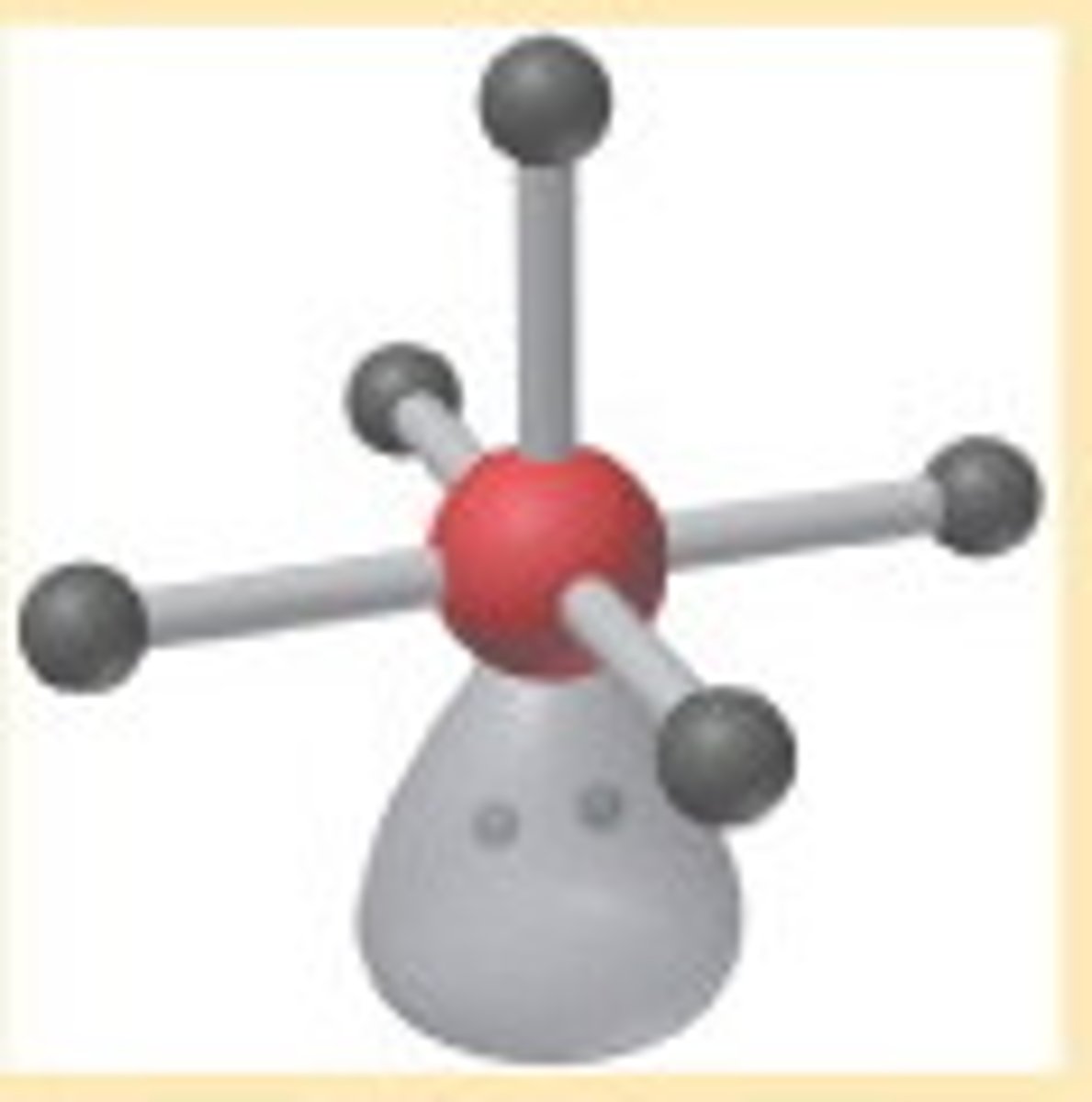
AX₄E₂
Square planar geometry, octahedral electron pair configuration.