Mineralogy Test 1 (lecture notes)
1/104
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
105 Terms
The process of light scattering and reflecting is part of the property called ___________.
Luster
When can color be used as a diagnostic property?
When chemical element causing the color (chromophore) is in mineral in a specific quantity
What is is called when an electron moves from an allowed orbit to another?
Quantum Jumping.
When does an electron acquire energy?
When jumping from lower orbit to higher one, or from higher temperature or electrical discharge
Which component of the X-ray tube is responsible for emitting the electrons that generate X-rays?
The cathode
Why is copper (Cu) commonly used as the target material in X-ray tubes for mineralogical analysis?
It produces Kα radiation at 1.5406 Å, ideal for resolving the interatomic spacing in most minerals
Two minerals have the same chemical formula but different crystal structures. What will their XRD patterns look like?
Completely different, because the atomic arrangements differ
The Miller indices (hkl) associated with a diffraction peak represent…
The specific set of lattice planes responsible for the diffraction
A broad and weak XRD peak in a mineral sample most likely indicates what?
An amorphous or poorly crystalline material.
Explain how XRD is used to identify minerals.
By comparing the diffraction pattern (peak positions and intensities) to reference patterns in a database
What could cause two different minerals to have similar XRD patterns, potentially leading to incorrect identification?
The minerals are isostructural (share the same crystal structure, even though their chemical compositions may vary)
Why do only certain planes in a crystal diffract X-rays at specific angles?
X-ray diffraction can only happen when the geometry is just right for the scattered waves to reinforce each other instead of cancel out (called Bragg’s Law)
What distinguishes X-ray diffraction (XRD) from X-ray fluorescence (XRF) in terms of interaction with minerals?
XRD (X-ray diffraction): measures how X-rays scatter off the planes in a crystal → gives information about the atomic arrangement and crystal structure.
XRF (X-ray fluorescence): measures secondary X-rays emitted when atoms are excited → gives information about the elements present and their concentrations.
What key law relates X-ray wavelength, lattice spacing, and diffraction angle?
Bragg’s law
Why don’t amorphous materials produce sharp peaks in XRD?
They lack long-range periodic order, so they show only broad humps in the diffraction pattern.
Why is random orientation of powder particles important in XRPD?
It ensures that all possible lattice planes satisfy Bragg’s condition and produce measurable reflections.
What are the two main types of X-ray spectra?
Continuous spectrum (white radiation) from electron deceleration.
Characteristic spectrum from electron transitions within the anode target.
What are the essential components of a powder x-ray diffractometer?
X-ray tube, incident beam optics (slits/filters), sample holder, goniometer, receiving optics, and detector.
Why is it important to have a smooth, flat, densely packed sample with randomly oriented grains in XRD?
To ensures accurate peak positions and intensities.
How is the radius ratio calculated and why is it important?
R=Rc/Ra ; predicts the coordination number (CN) of a cation.
What is coordination number (CN)?
The number of nearest-neighbor anions surrounding a cation in a crystal structure.
What is Pauling’s First Rule?
Radius Ratio Principle – CN depends on the cation/anion size ratio and determines the geometry of the coordination polyhedron.
What is a principal quantum number (n)?
It indicates an electron’s main energy level or shell.
What controls most physical and chemical properties of minerals?
Bond type and bond strength
Which type of bond is this?
→ Atoms share electrons directionally; strongest bond type; creates hard, high-melting, non-conductive minerals (e.g., diamond, quartz).
Covalent bond
Which type of bond is this?
→ Electrostatic attraction between cations and anions; common in minerals; bond strength depends on charge and interionic distance; forms between a metal and nonmetal.
Ionic bond
Give the equation for the force of attraction in ionic bonds.
Coulomb’s law: F=(q1*q2)/d2
Which type of bond is this?
→ Valence electrons form a shared “sea,” giving high electrical/thermal conductivity and malleability (native metals like Cu, Au).
Metallic bond
Which type of bond is this?
→ Weak attraction from fluctuating electron distributions forming temporary dipoles (e.g., layers in graphite).
van der Waals bond
What is interatomic distance and how is it measured?
Distance between cation and anion centers; measured by X-ray diffraction.
d=r(cation)+r(anion)
Fill in the names of each CN:
CN 2 (______): R < 0.155
CN 3 (______): 0.155–0.225
CN 4 (________): 0.225–0.414
CN 6 (_________): 0.414–0.732
CN 8 (_______): 0.732–1.0
CN 12 (__________): ≈1.0
linear
triangular
tetrahedral
octahedral
cubic
close packing
What are the two main types of close packing?
Hexagonal close packing (hcp, ABAB) and cubic close packing (ccp/fcc, ABCABC).
What are tetrahedral sites?
4-fold interstices formed when an atom rests in the depression of three atoms from one layer and one from the next.
What is Pauling’s Second Rule?
Electrostatic Valency Principle – The total bond strength to an anion equals its charge.
Bond strength p = z/CN
z = cation charge
What is Pauling’s Third Rule?
Sharing of edges or faces between cation polyhedra decreases stability due to cation–cation repulsion.
What three factors determine bond strength?
Ionic charge, coordination number, and packing efficiency.
Why is graphite soft despite covalent bonding?
Strong bonds within sheets but very weak van der Waals bonds between sheets allow easy cleavage.
What is the most basic object (atom, ion group, or molecule) that repeats in space to form a crystal?
A motif (node)
What is a symmetry element?
A geometric entity (point, line, or plane) about which a symmetry operation is performed.
What is translational symmetry?
Repetition of a motif at regular intervals to produce an infinite periodic lattice.
What is the smallest repeat unit that, by translation only, generates the entire lattice of a crystal?
A unit cell
What are the rules for choosing the correct unit cell?
Choose the smallest cell, most orthogonal, highest symmetry, and primitive (non-centered) when possible.
What are imaginary reference lines (x, y, z) inside a crystal lattice that define directions and orientations?
crystallographic axes.
List the seven crystal systems with key parameters.
Triclinic: a ≠ b ≠ c; α ≠ β ≠ γ ≠ 90°
Monoclinic: a ≠ b ≠ c; α = γ = 90°, β ≠ 90°
Orthorhombic: a ≠ b ≠ c; α = β = γ = 90°
Tetragonal: a₁ = a₂ ≠ c; α = β = γ = 90°
Hexagonal: a₁ = a₂ = a₃ ≠ c; β = 90°, γ = 120°
Rhombohedral (Trigonal): a₁ = a₂ = a₃; α = β = γ ≠ 90°
Isometric (Cubic): a₁ = a₂ = a₃; α = β = γ = 90°.
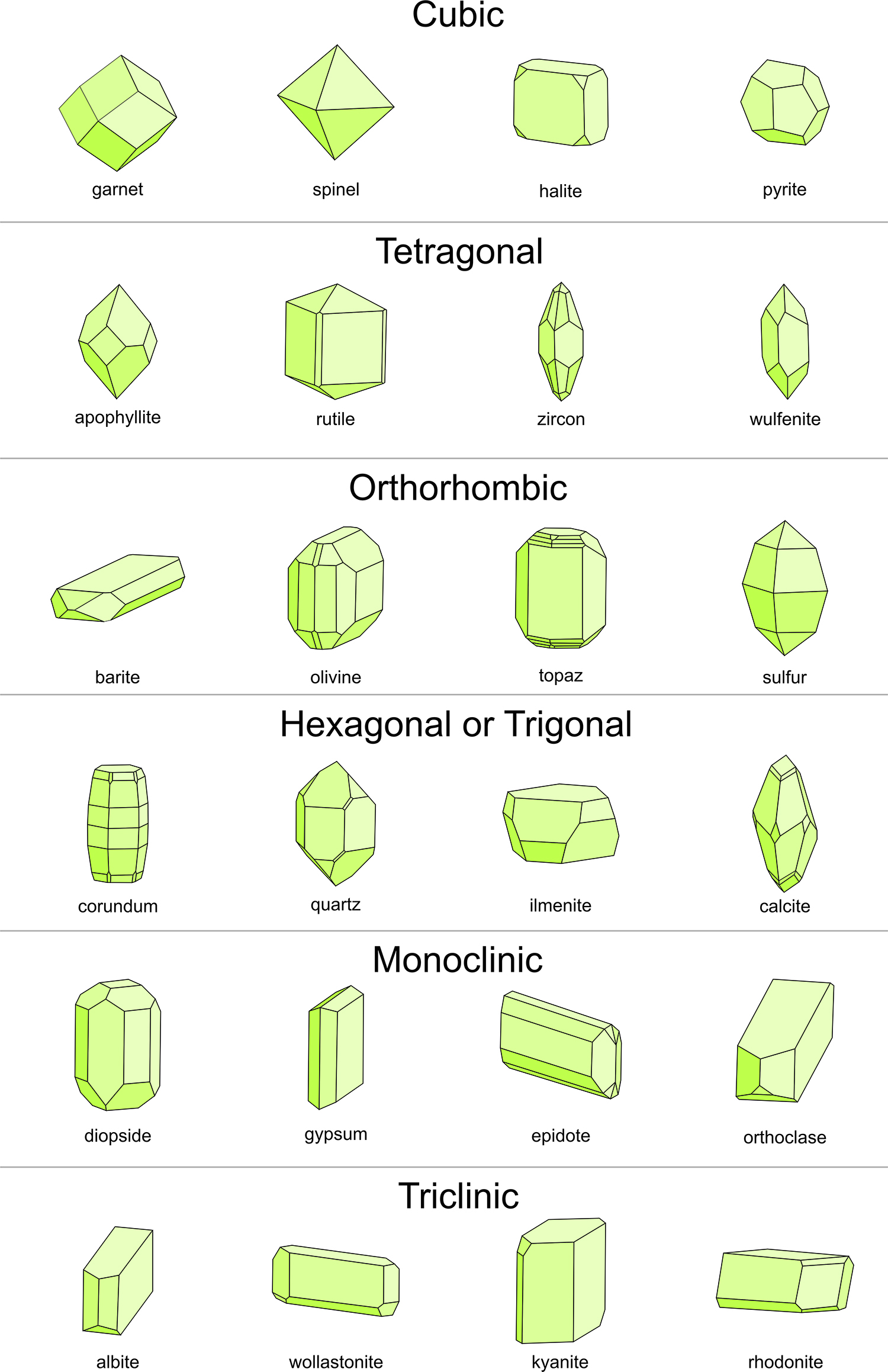
What determines a crystal system?
The geometry of the unit cell—edge lengths (a, b, c) and interaxial angles (α, β, γ).
Explain a 1-fold rotation axis.
Requires 360° rotation to repeat; effectively no rotational symmetry.
Explain a 2-fold rotation axis.
Object repeats after 180° rotation (two times in 360°).
Explain a 3-fold rotation axis.
Object repeats after 120° rotation (three times in 360°).
Explain a 4-fold rotation axis.
Object repeats after 90° rotation (four times in 360°).
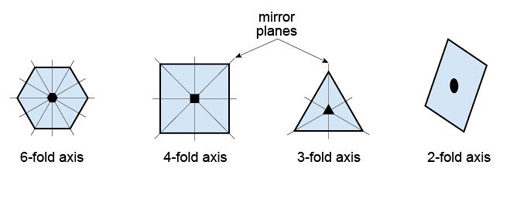
Explain a 6-fold rotation axis.
Object repeats after 60° rotation (six times in 360°).
Why are 5-fold or 7-fold axes not allowed in crystals?
Such symmetries cannot fill 3D space without leaving gaps.
Why is the smallest, most symmetric unit cell preferred?
It simplifies lattice description and ensures accurate symmetry representation.
What is the set of all symmetry operations (rotation, reflection, inversion, rotoinversion) that leave at least one point unchanged and describe a crystal’s external symmetry?
Point groups.
What do the 14 Bravais lattices represent?
The only possible 3D arrangements of points that preserve periodicity, symmetry, and lattice uniqueness.
Which crystal system is this?
→ Minimal symmetry; no constraints on angles or axes; H–M symbols 1 or 1̅.
Triclinic system
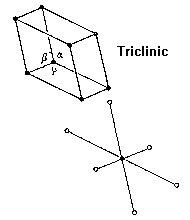
Which crystal system is this?
→ A single 2-fold axis or a mirror plane; examples: 2, m, 2/m.
Monoclinic system
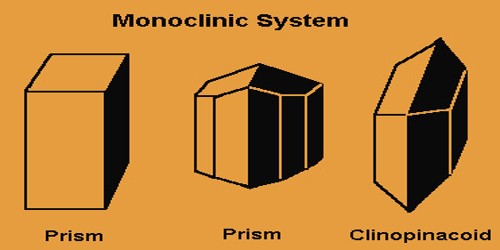
Which crystal system is this?
→ Three mutually perpendicular 2-fold axes or mirror planes; examples: 222, mm2, 2/m 2/m 2/m.
Orthorhombic system
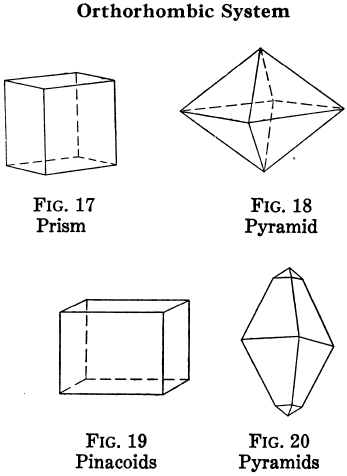
Which crystal system is this?
→ One 4-fold rotation or rotoinversion axis along c; examples: 4, 4/m, 422, 4mm.
Tetragonal system
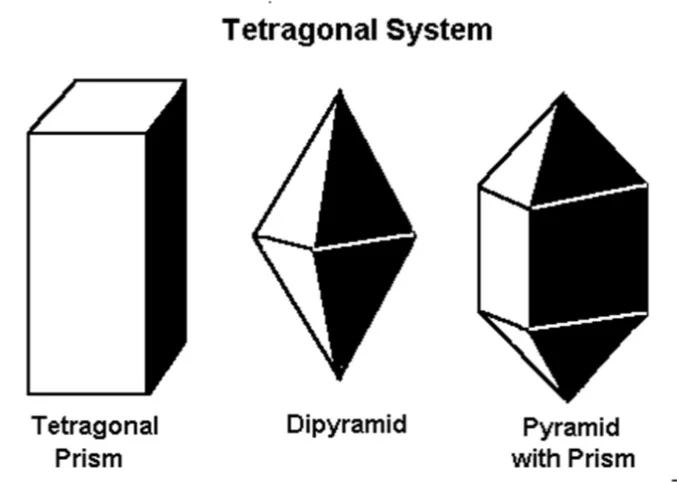
Which crystal system is this?
→ A: One 3-fold rotation or rotoinversion axis; examples: 3, 32, 3m.
Trigonal system
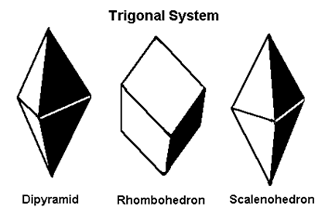
Which crystal system is this?
→ One 6-fold rotation or rotoinversion axis; examples: 6, 6/m, 622, 6mm.
Hexagonal system
What crystal system is this?
→ Four 3-fold axes along cube diagonals, plus multiple 2- or 4-fold axes; examples: 23, 432, 43m.
Isometric (cubic) system
What is the overall external shape of a crystal (e.g., cubic, prismatic, pyramidal), influenced by growth conditions, called?
Crystal habit.
Ex: Dendritic, Fibrous, Prismatic, Bladed, Reniform

What is the relationship between point groups and space groups?
Point groups describe external symmetry; space groups combine point group symmetry with translational symmetry to describe the internal lattice.
What is the set of integers (h k l) that describe the orientation of a lattice plane by its intercepts with crystallographic axes?
Miller indices
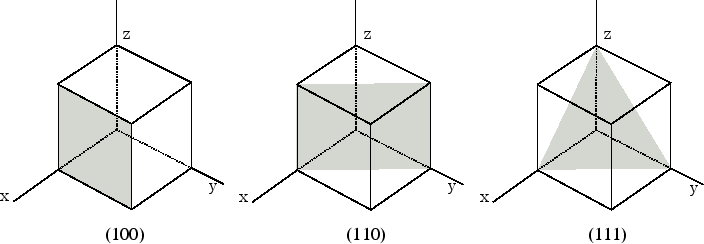
What does a zero in a Miller index mean?
The plane is parallel to that axis.
Summarize the hierarchy of crystal symmetry.
→ Bravais lattices (14) define basic lattice types
→ Point groups (32) add rotational/mirror/inversion symmetry
→ Space groups (230) include translation plus screw and glide operations.
What are imperfections in the crystal lattice introduced during growth, phase transformations, or deformation?
Crystalline defects
What is a type of point defect in which a lattice site is missing an atom or ion?
Vacancy defect
What is a type of point defect in which a missing cation is balanced by a missing anion to maintain charge neutrality in ionic crystals?
Schottky defect
What is a type of point defect in which a cation is displaced from its normal site and relocated elsewhere in the structure?
Frenkel defect.
What is a type of point defect in which a foreign cation is located in the structure, balanced by charge compensation elsewhere?
Interstitial defect
What is a type of point defect in which a foreign cation substitutes for a normal cation in the lattice?
Substitution defect
What are impurity defects?
Point defects caused by foreign atoms/ions entering interstices or replacing host atoms.
How do impurities affect minerals?
They can alter chemical composition and significantly change mineral color.
What is material trapped within a mineral during formation called?
Inclusion

What is the difference between an impurity and an inclusion?
Impurity = mixed/diffused into the lattice, cannot usually be seen because it is part of the internal crystal structure
Inclusion = distinct clump of material inside the mineral that can be seen.
What is a type of line defect in which a part of a crystal slips over another along defined slip planes?
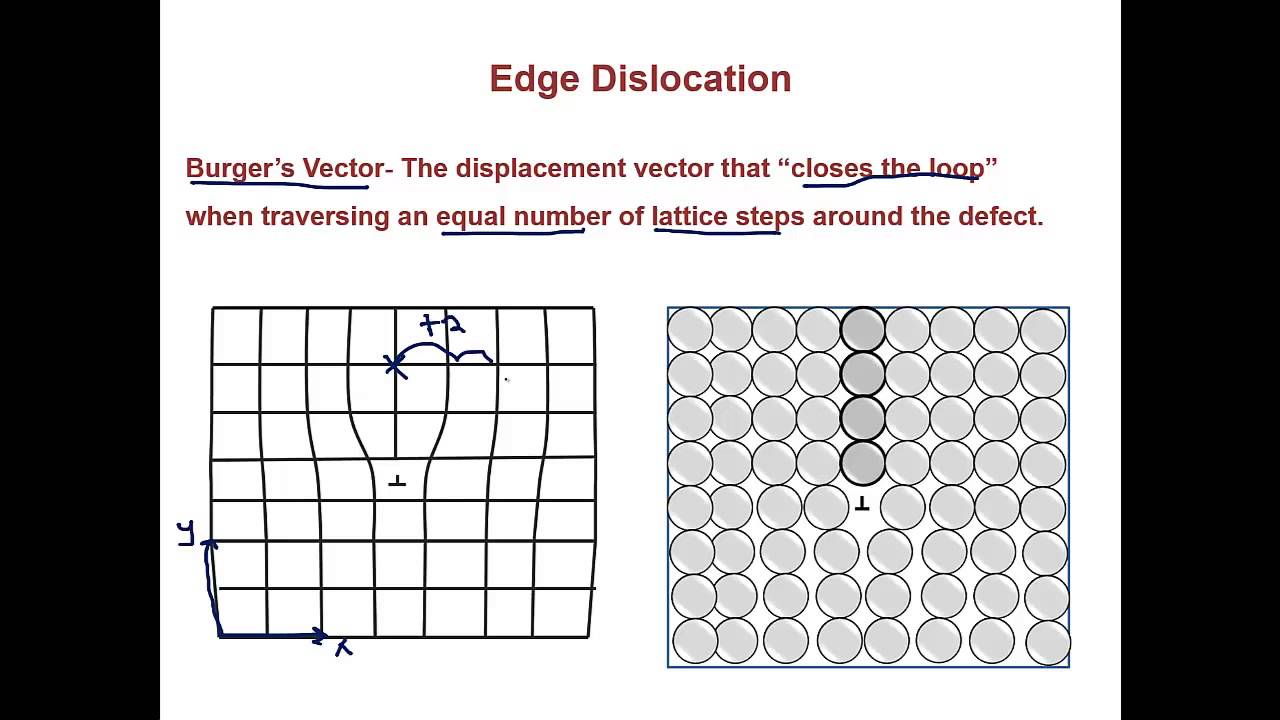
Dislocation
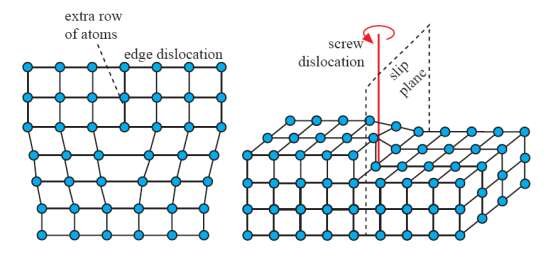
What is the vector that represents the magnitude and direction of lattice distortion from a dislocation?
The burgers vector
What is it called when two parts of a crystal intergrow, sharing a lattice plane (twin plane) or direction (twin axis)?
Twinning (planar defect)
When do crystal twins form?
During crystal growth, phase transformations, or deformation.
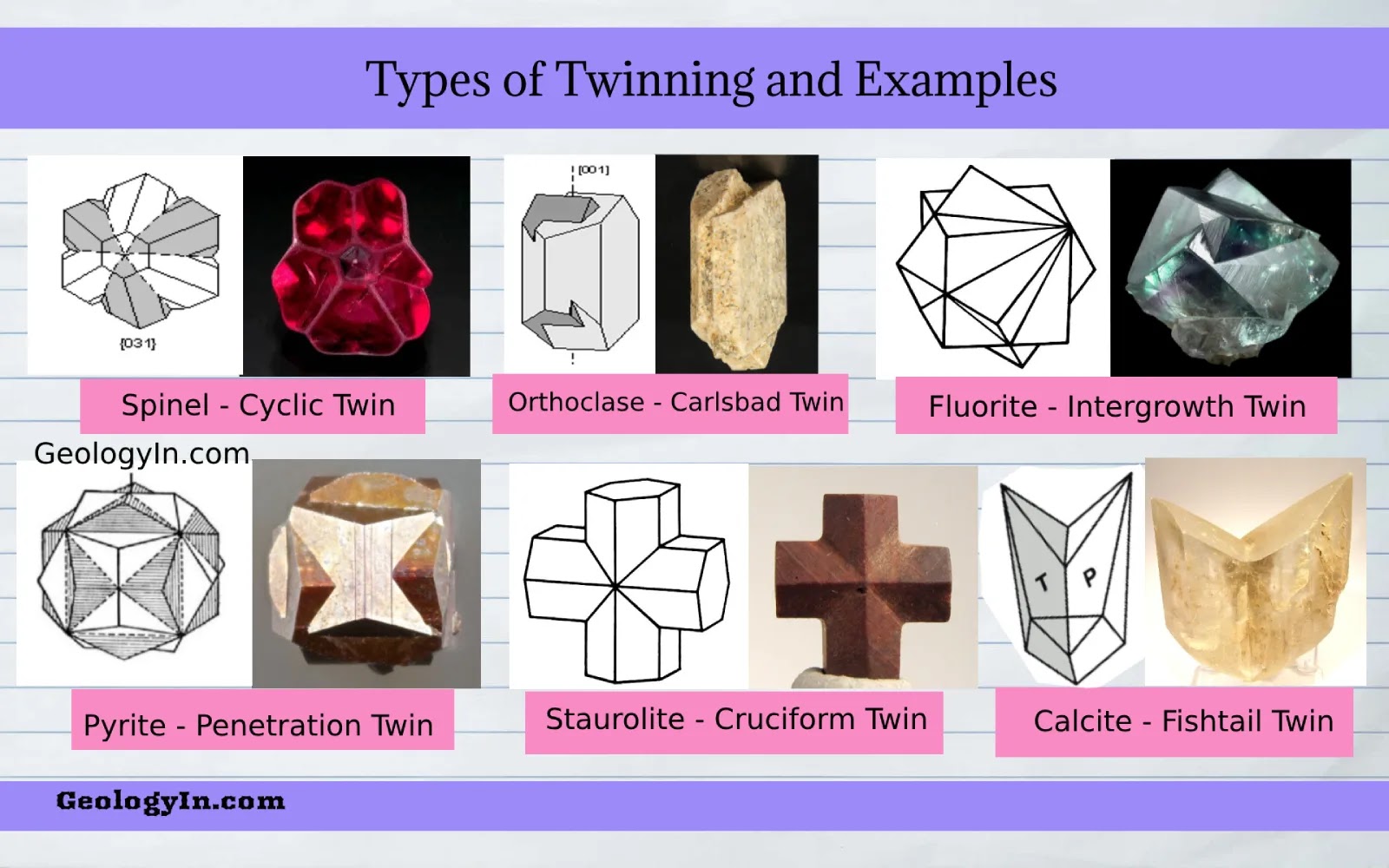
What are contact twins in crystals?
Host and twin share a rational crystal plane (compositional plane).
What are penetration twins in crystals?
Patchy intergrowths of two crystals with no obvious plane, but related by a twin law.

What is the Albite law of twinning in crystals?
Common in plagioclase; albite-anorthite twinning; diagnostic for identification.
What is the Pericline law of twinning?
Occurs when orthoclase/sanidine transforms to microcline; often combined with Albite law to form “tartan twinning.”
What is structural damage in crystals caused by radioactive decay (e.g., zircon with U/Th damaging biotite, forming pleochroic halos) called?
Radiation defects
When does an electron jump from a lower orbit to a higher orbit?
When an electron absorbs energy
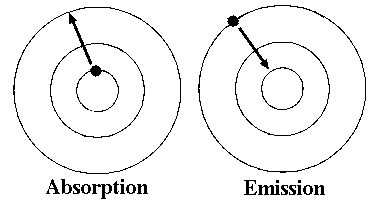
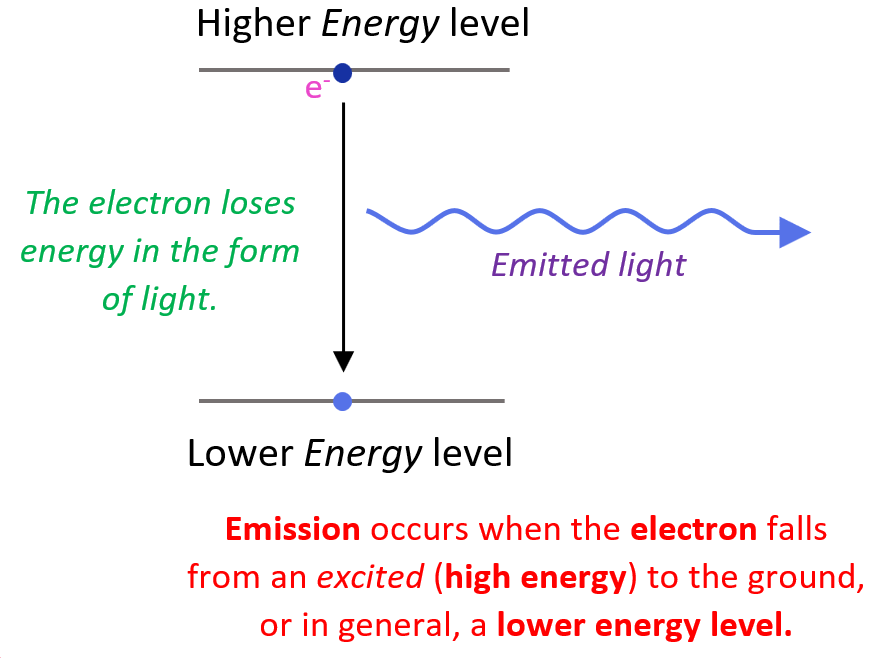
When does an electron fall to a lower orbit and emit light as a photon?
When an electron releases energy
What are color-causing elements or ions in minerals that absorb specific wavelengths of light?
Chromophores
Which two ions commonly act as chromophores?
Fe³⁺ and Cr³⁺
Why do transition metals influence color strongly?
Their 3d-orbitals have unpaired electrons that absorb visible light.
What is the electron transfer between adjacent cations of different oxidation states called?
Molecular orbital (charge transfer) transition
What causes a color center in a crystal?
Structural defects such as vacancies or trapped electrons.
What are some minerals that have color centers?
Amazonite, amethyst, fluorite, smoky quartz, diamond, topaz, halite.

What is the immediate (in nanoseconds) light emission after absorbing UV/visible radiation called?
Fluorescence

What is delayed emission caused by electrons trapped in metastable states called?
Phosphorescence.
What is the Schiller effect?
The color effect in labradorite caused by exsolution lamellae acting as diffraction gratings.


What causes the interesting play-of-color in opal?
Silica spheres arranged in arrays that diffract visible light.
What is an ideal formula?
Give some examples.
The composition of a chemically pure mineral.
Al₂O₃ (corundum), CaCO₃ (calcite), Mg₂SiO₄ (forsterite), FeS₂ (pyrite), NaCl (halite).
How is charge balance checked in mineral formulas?
By multiplying each ion’s charge by its stoichiometric coefficient and summing charges.
Is diopside (CaMgSi₂O₆) charge balanced?
Yes
— total cation charges (+12) equal total anion charges (−12).