Electron Configurations, Electronic Configuration
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
32 Terms
Hydrogen
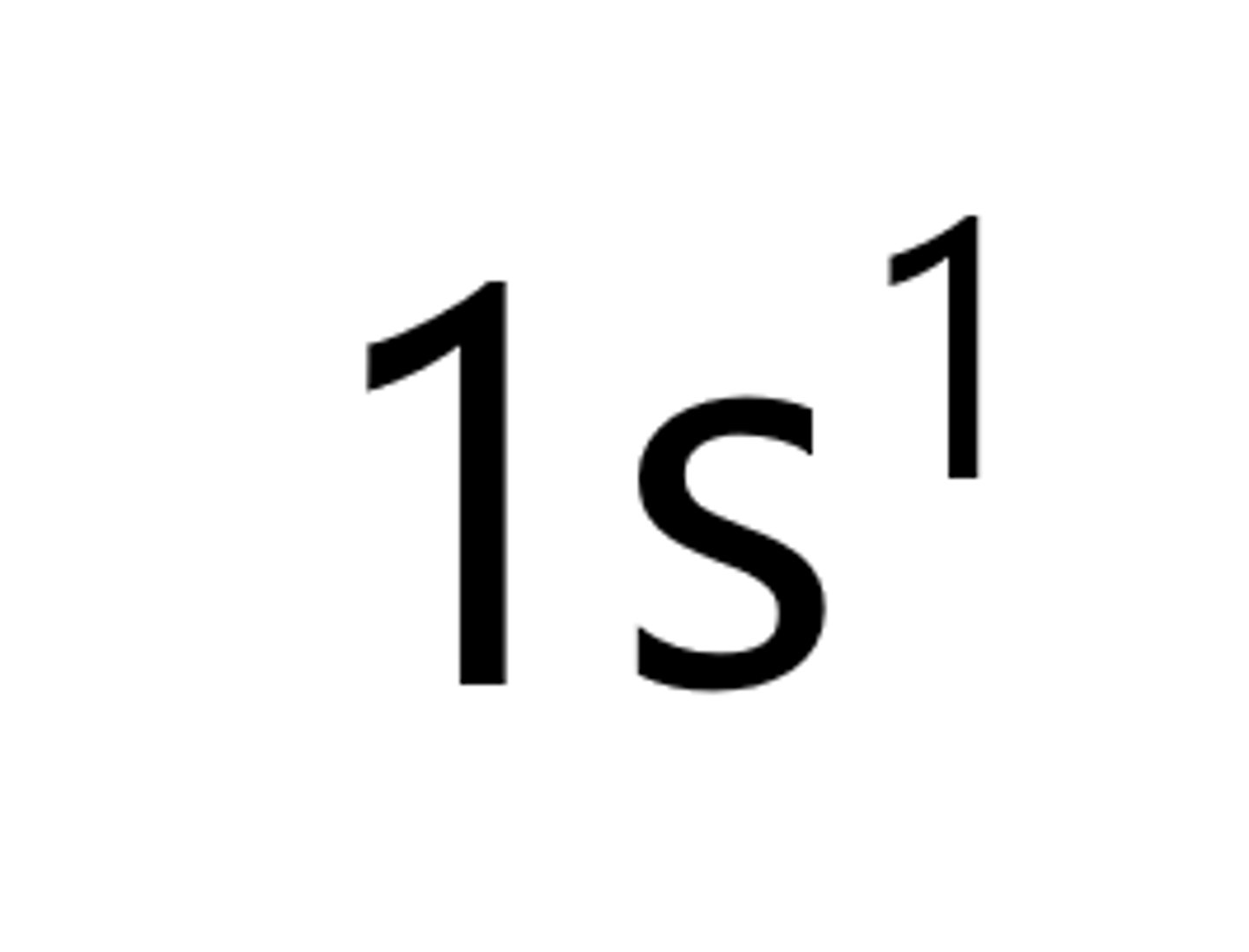
Lithium
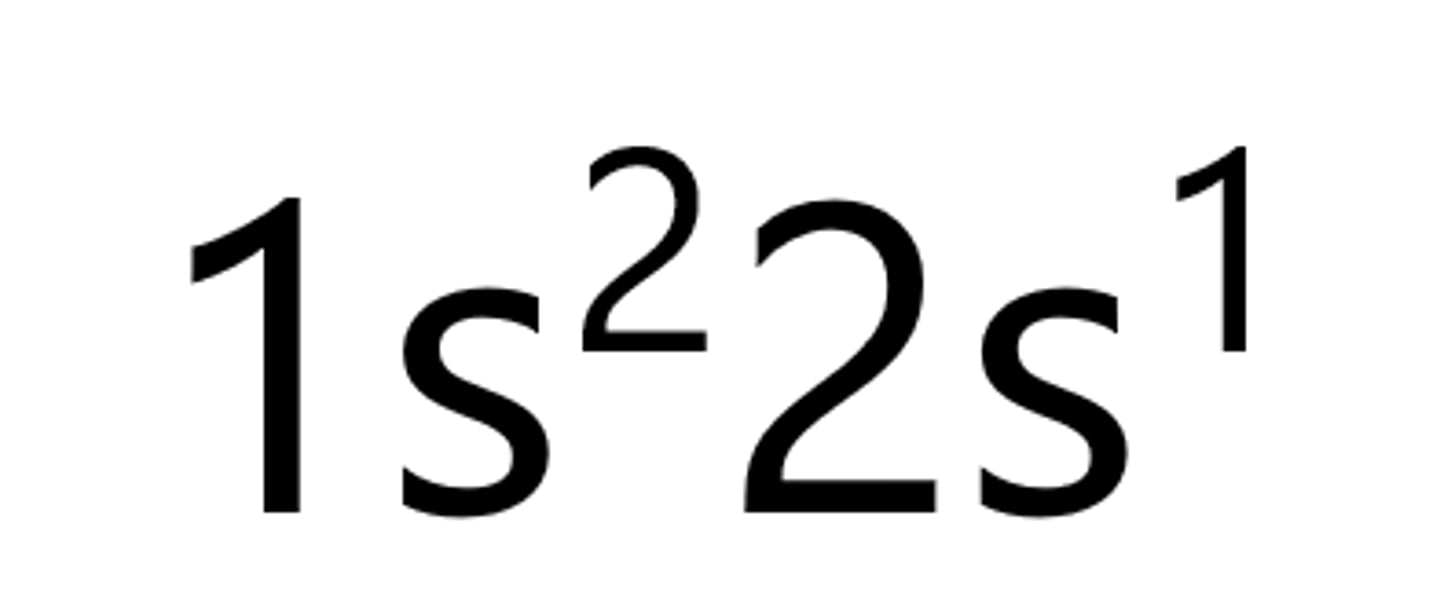
Beryllium
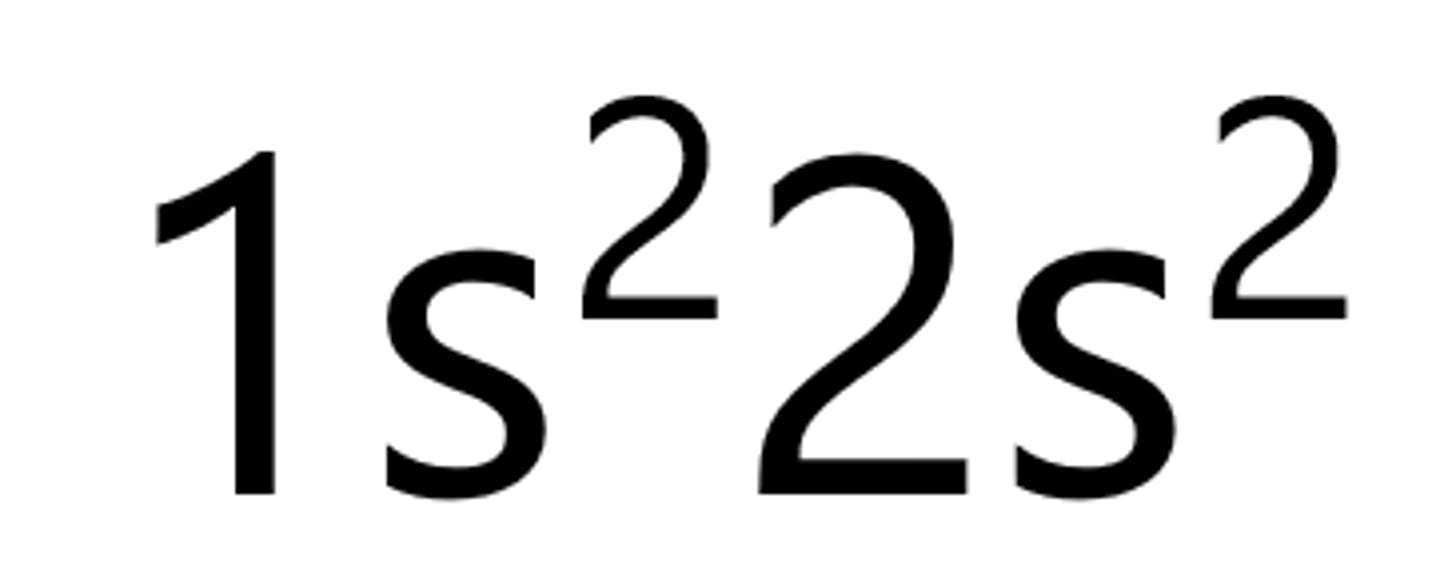
Boron
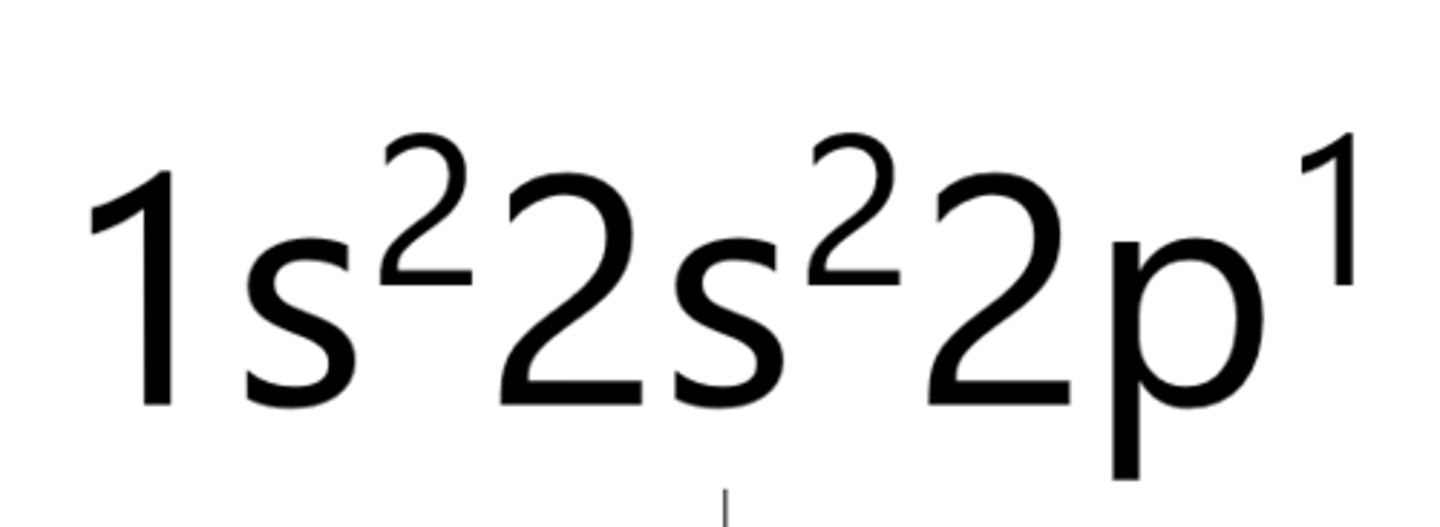
Oxygen
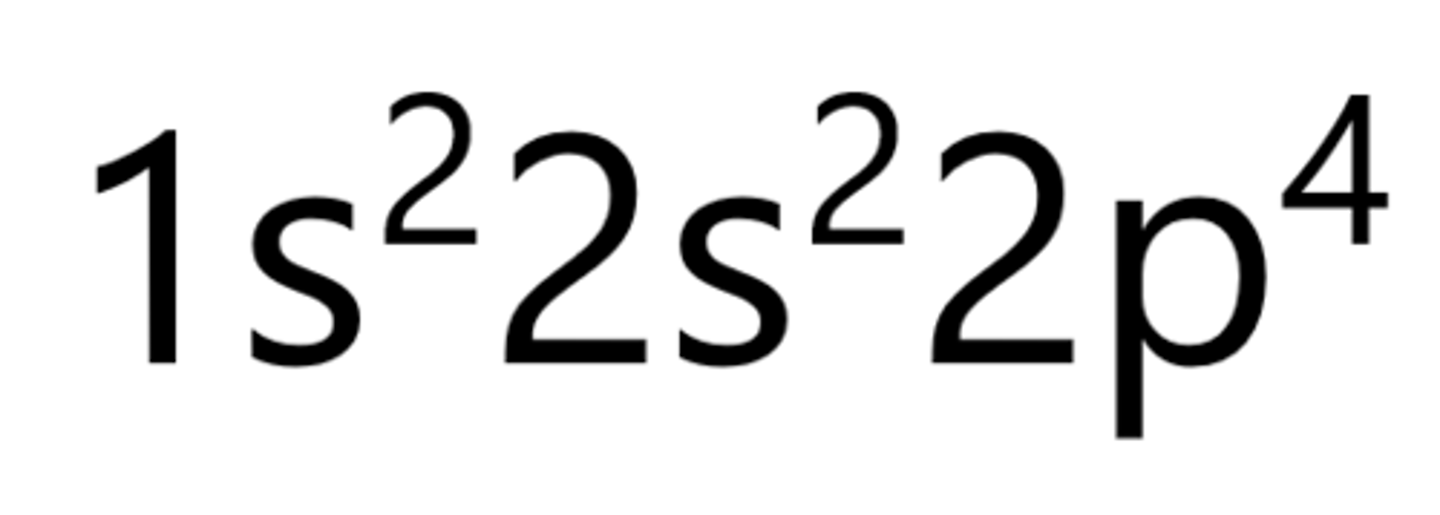
Sodium
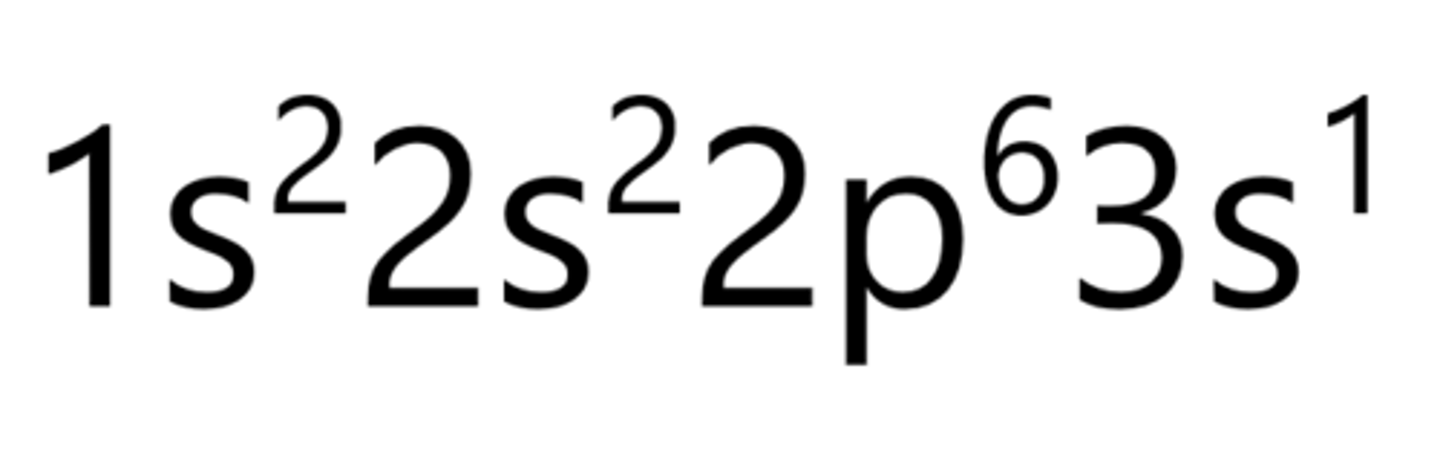
Magnesium
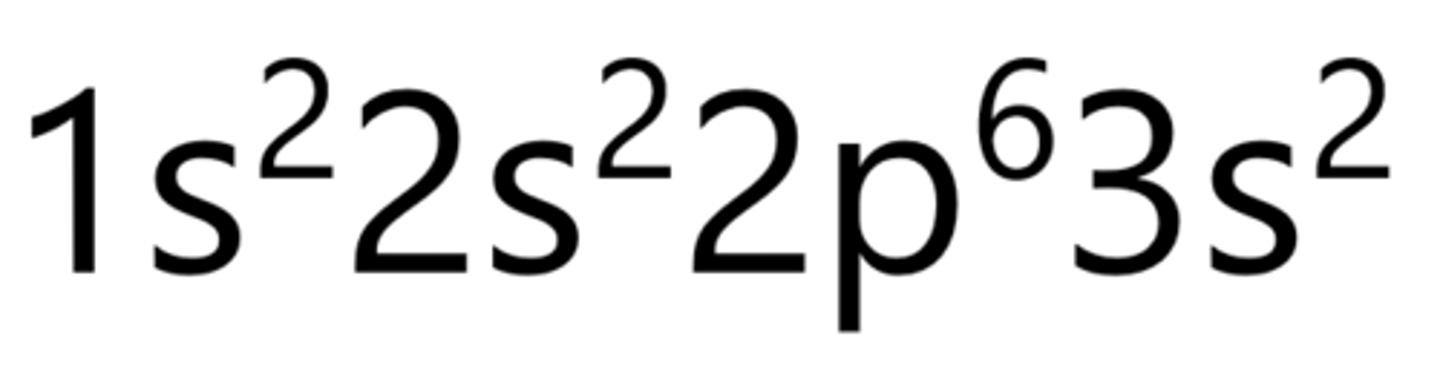
Aluminum
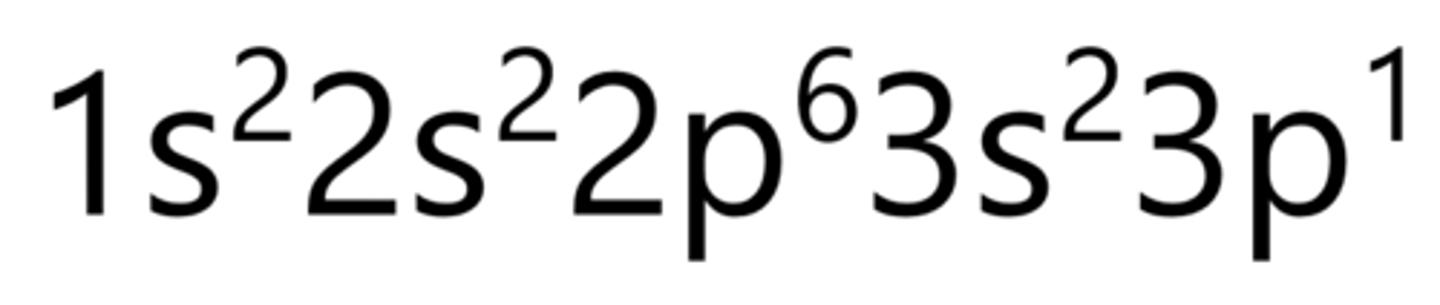
Silicon
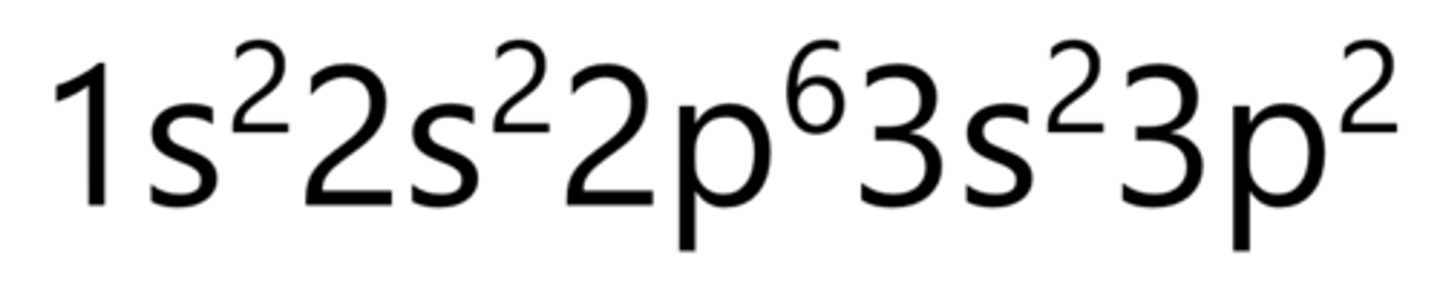
Sulfur
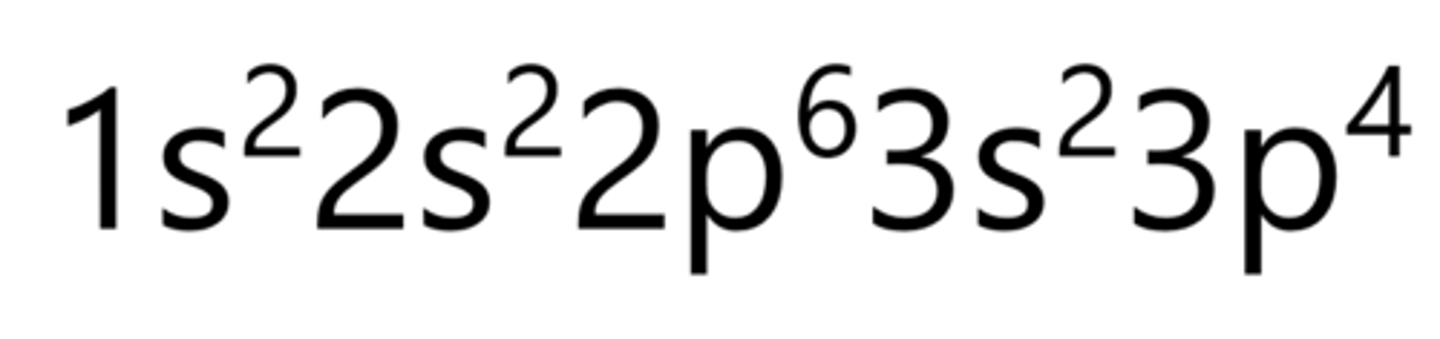
Argon
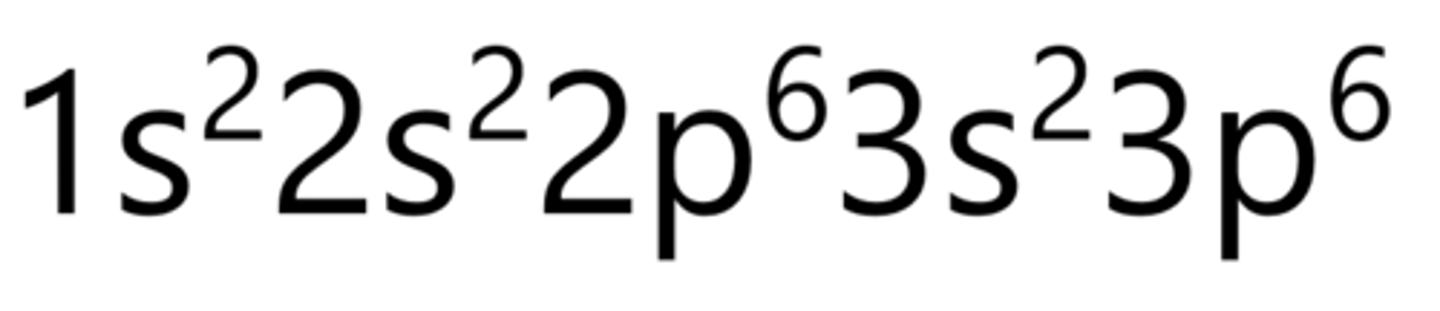
Scandium
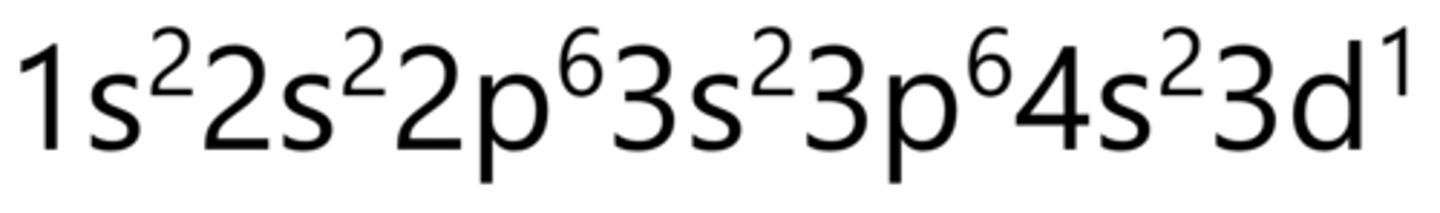
Chromium
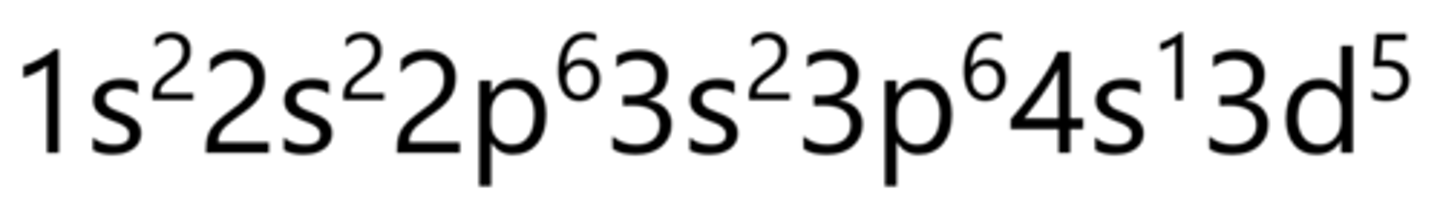
Iron
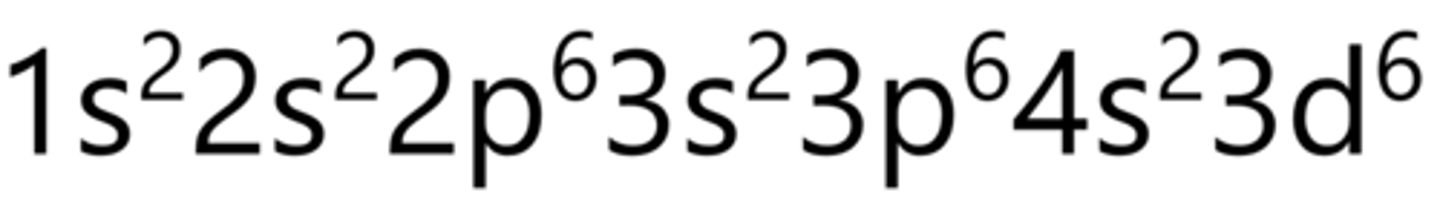
Cobalt
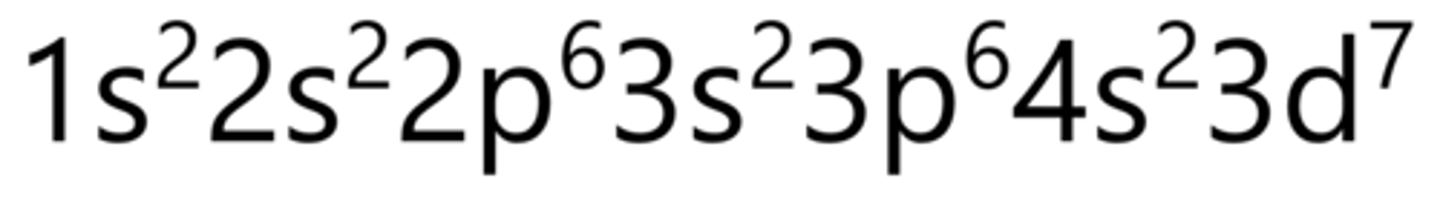
Copper
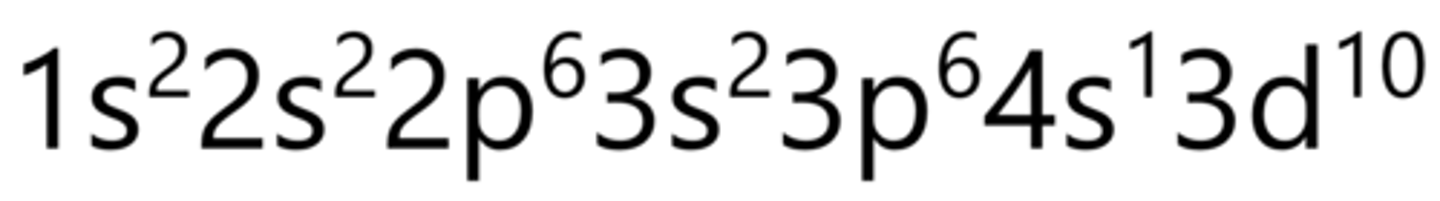
Germanium
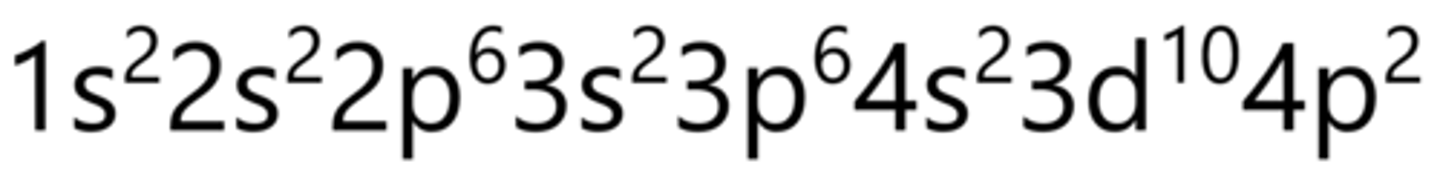
Selenium
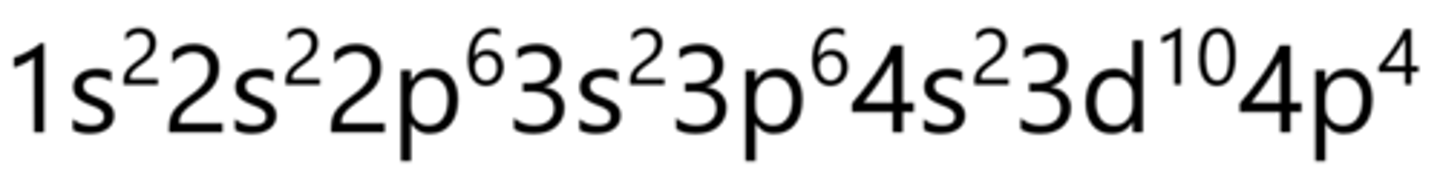
Bromine
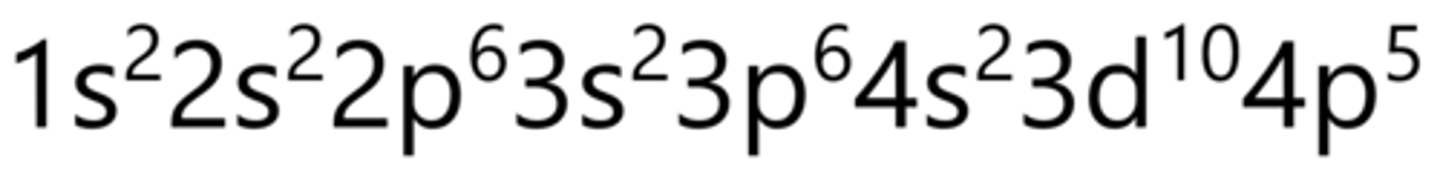
Krypton
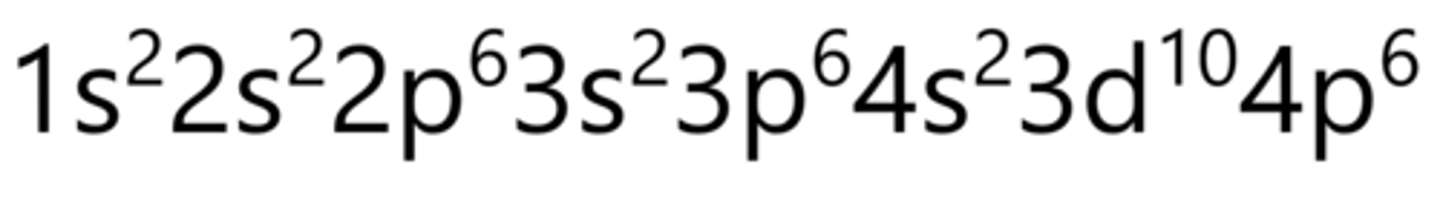
Zirconium
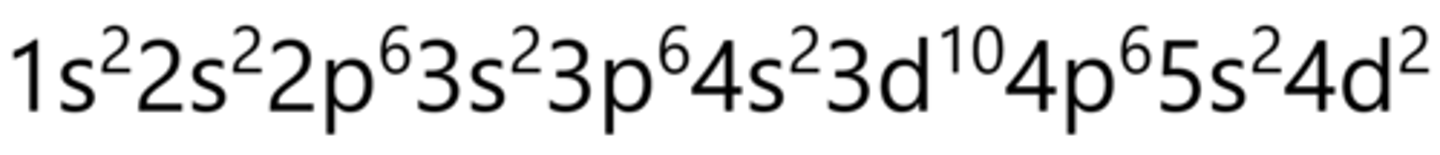
Hund's Rule
Every orbital is singly occupied with one electron before its doubled
names of sublevels
s, p, d, f
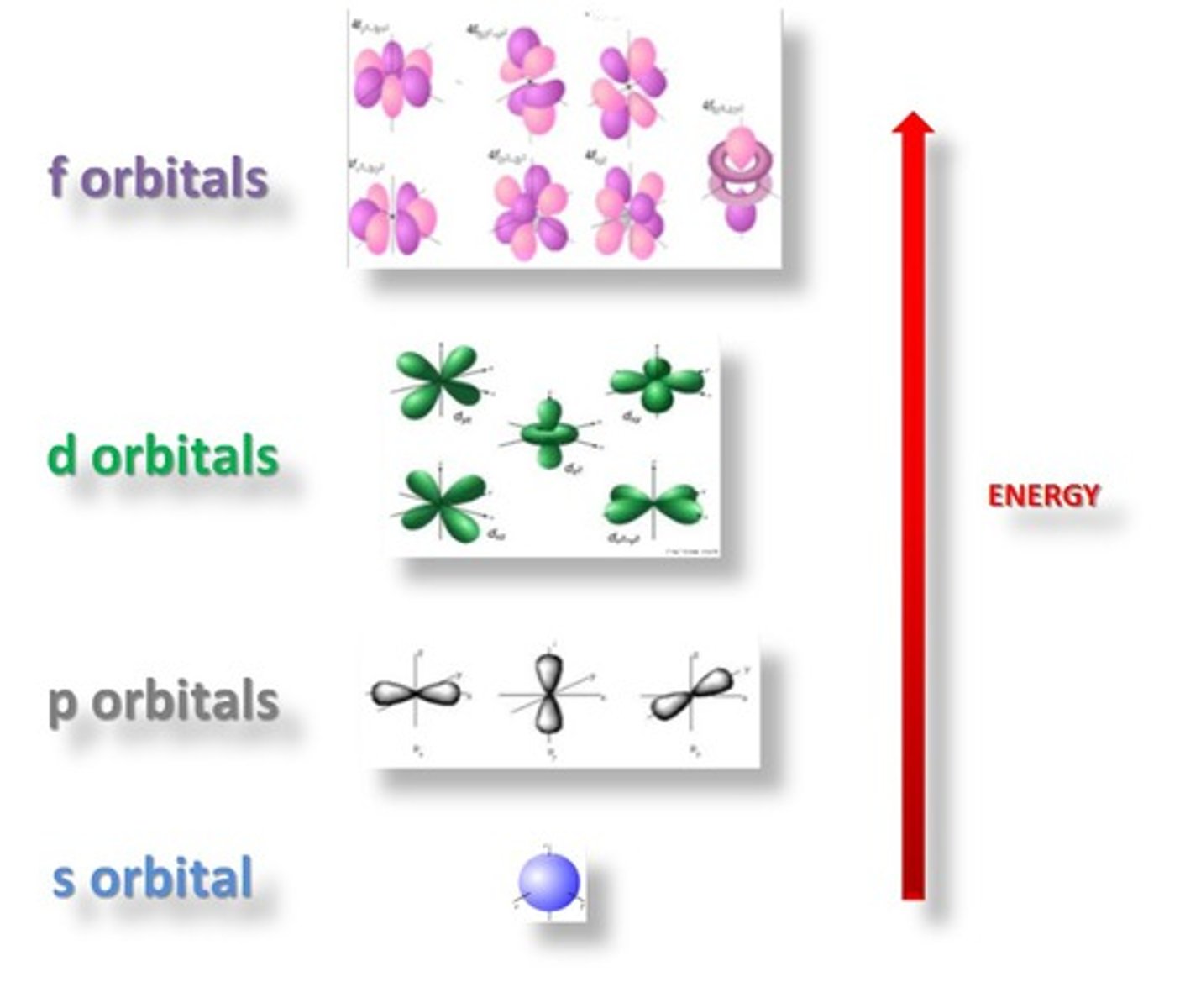
1s sublevel
sphere shape
closest to the nucleus
least amount of energy
"p" sublevel shape
teardrop/ dumbell
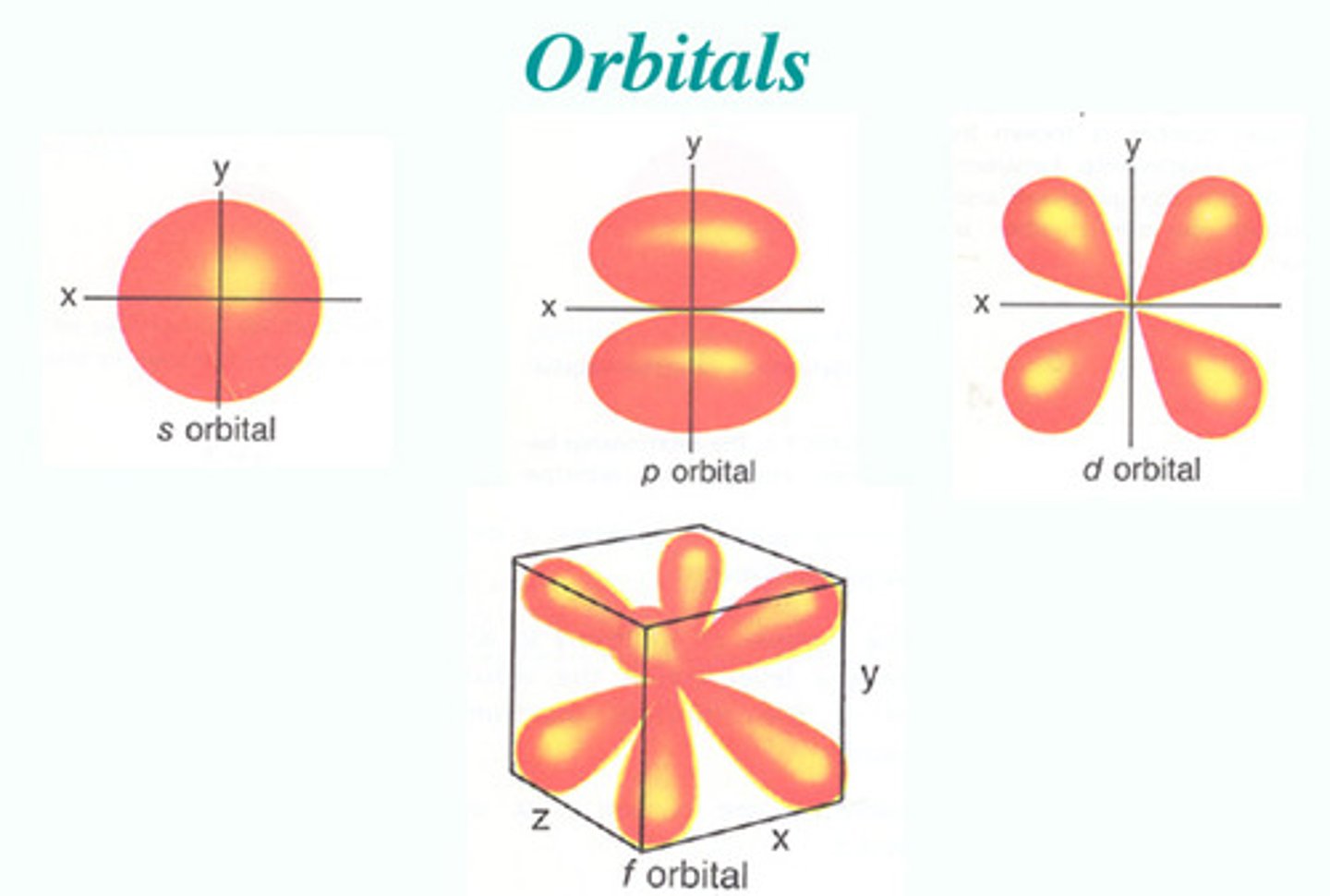
how many electrons can each orbital hold?
2
how many orbitals are in an "s" sublevel?
1 orbital, so it can hold 2 electrons
how many orbitals are in a "p" sublevel?
3 orbitals, so it can hold 6 electrons
how many orbitals are in a "d" sublevel?
5 orbitals, so it can hold 10 electrons
how many orbitals are in a "f" sublevel?
7 orbitals, so it can hold 14 electrons
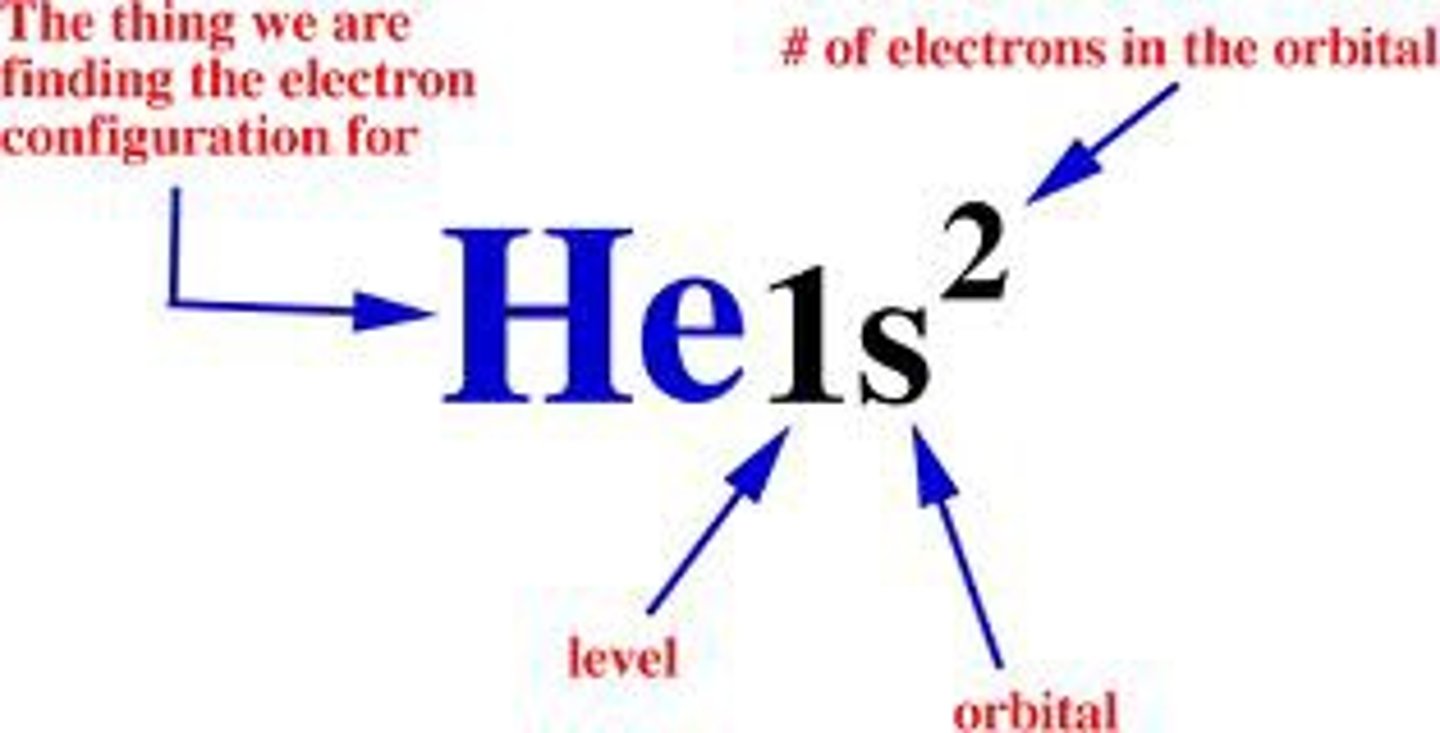
electron configuration notation
number= energy level
letter= sublevel
little number= number of electrons in the sublevel
the 4s sublevel comes BEFORE ___
the 3d sublevel
goes in order: 3s, 3p, 4s, 3d, 4p, 4d, 4f