Lecture 47: Liquid dosage forms for oral administration- solutions
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
what are the advantages of pharmaceutical oral solutions?
easier to swallow(old ppl, patients)
faster therapeutic response(drug instantly available for absorption)
homogenous system, uniform distribution, no issues with phase separation such as with emulsions and suspensions
reduced irritation, immediately diluted by gastric contents
masks taste of bitter therapeutic agents
what are the disadvantages of pharmaceutical oral solutions?
problems associated with manufacture, transport(breaking container), administration
growth of microorganism
poorer stability of excipients in aq sol vs a solid dosage form(ie surfactants, preservatives, colours, flavours)
shorter shelf life
less dose accurate (can fix by using 5ml spoons)
taste
unsuitable for drugs which are chemically unstable in presence of water
expensive to ship, bulky for patient to carry(weight)
what are some challenges to making oral solutions?
Homogeneity in the formulation, being all the same composition
how does the aqueous solubility of the drug affect the formulation at specific pH’s?
high solubility at selected pH: drug is readily incorporated into the vehicle
Moderate solubility at selected pH: drug solubility in the formulation needs to be enhanced using co-solvents, related methods
low solubility at selected pH: alternative dosage form should be used: e.g. suspension
what are the features of the vehicles used in oral solutions?
they are made from purified water USP
low cost and toxicity
tap water shouldnt be used as it has chemical incompatibilities
cant be used for parenteral formulations as they have to be sterile
how is purified water prepared?
distillation
ion exchange methods
reverse osmosis
the solid residue obtained after evaporation <1mg/100ml of evaporated sample
How can we increase the solubility of drug within formulation?
use cosolvents:
glycerol can be used and it has cosolvency properties due to 3 OH groups
alcohol: ethanol can be used
propylene glycol USP
poly(ethylene glycol): repeating units of ethylene oxide
lower MW grades (PEG200, 400) are preferred in pharmaceutical solutions
surface active agents and complexation can be used too
what are the different types of excipients in pharmaceutical oral solutions?
Buffers
sweetening agents
viscosity enhancing agents
what are buffers used for plus examples:
control pH
acetates(1-2%), citrates(1-5%), phosphates(0.8-2%)
what are sweetening agents used for plus examples:
they increase the palatability of drugs
eg. sucrose, glycerol, sorbitol, aspartame
what are viscosity-enhancing agents used for plus examples:
non-ionic: methylcellulose(cellulose derivatives)
ionic hydrophilic polymers: sodium carboxymethylcellulose(anionic)
some liquid formulations dont need viscosity inhancing agents(syrups: inherent viscosity)
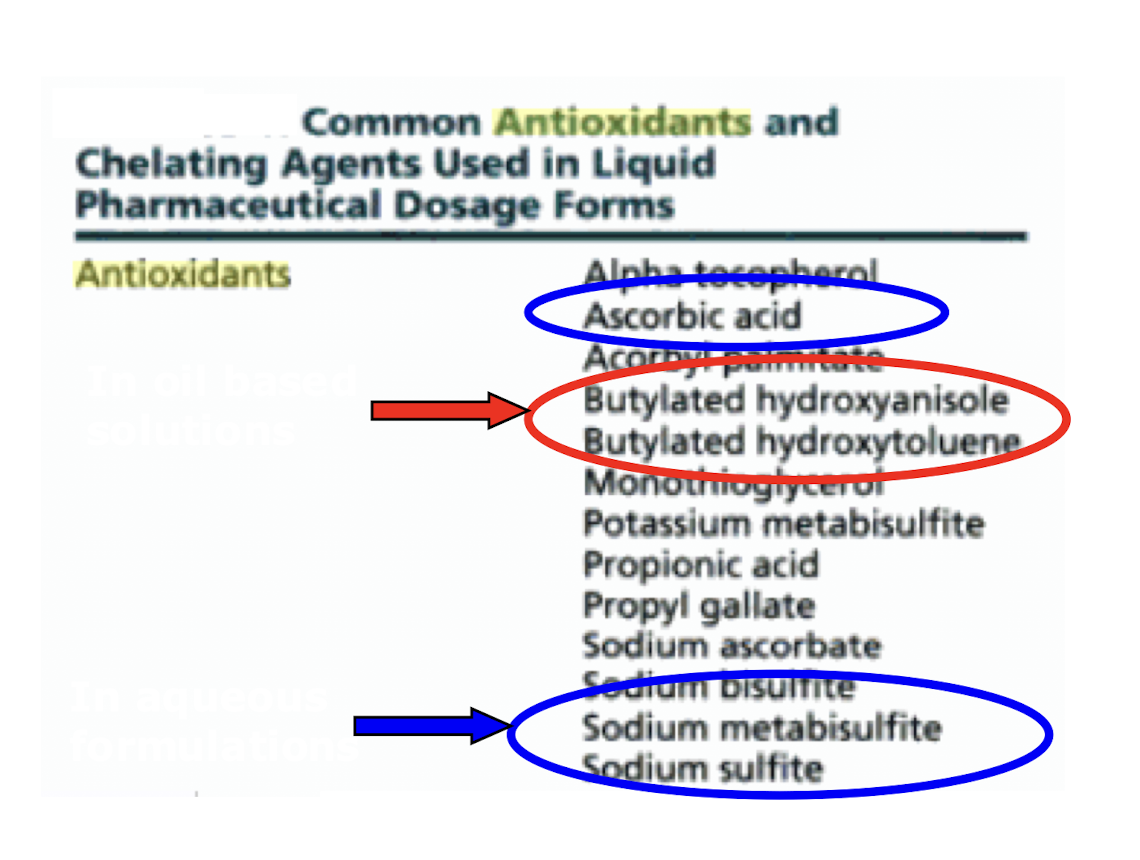
what are antioxidants?
redox systems that exhibit increased oxidative potential than drug OR
compounds that inhibit free radical induced drug decomposition
how are antioxidants used?
some drugs are susceptible to chemical degradation by oxidation, so we add antioxidants to them
they increase the stability of the drug
they are oxidised(degraded) instead of the drug which protects the drug from decomposition
they can be water soluble or water insoluble
what are preservatives and what are their properties?
preservatives control the microbial bioburden of the formulation
properties:
they have a broad spectrum of antimicrobial activity( G+, G-, bacteria, fungi)
chemically and physically stable over the shelf life of the product
low toxicity
what are examples of preservatives we use?
benzoic acid and salts (0.1-0.3%)
sorbic acid and its salts(0.05-0.2%)
alkyl esters of parahydroxybenzoic acid(0.001-0.2%); combination enhancement of the antimicrobial spectrum
why is the preservative efficacy in oral solutions important and how can it be affected?
the correct form of preservative needs to be available at the required conc to inhibit microbial growth. This is known as the Minimum inhibitory concentration: MIC(minimum conc to inhibit microbial activity)
the conc of preservative may be affected by other excipients and formulation pH:
the pH of formulation
the presence of micelles
the presence of hydrophilic polymers
the unionised form of the preservative is the one with the antimicrobial properties.
the preservatives diffused across the outer membrane of the microorganism and eventually into the cytoplasm. the cytoplasm has neutral conditions which allow the preservatives to dissociate which acidifies the cytoplasm and inhibits microbial growth
the fraction of acidic preservative at a particular pH can be calculated with the henderson-hasselbalch eq
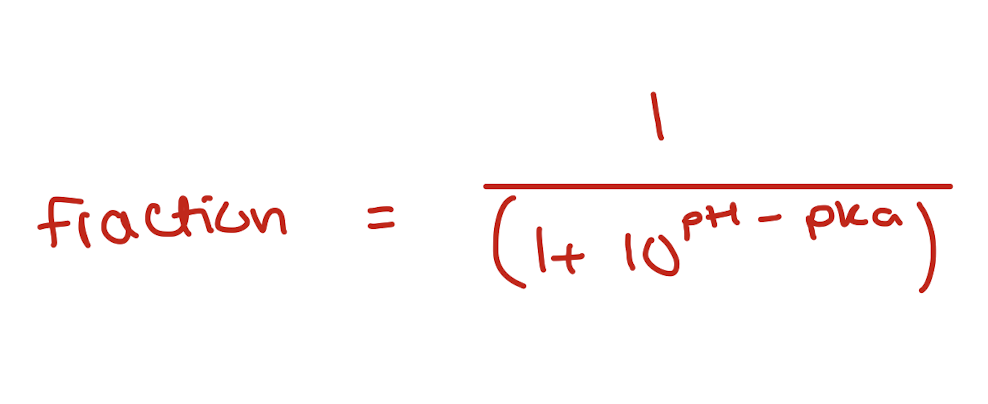
how does the presence of micelles affect preservatives?
micelles solubilise lipophilic drugs
the lipophilic properties(unionised form of acid preservatives) may partition into the micelle which then decreases the available and effective conc of preservative in solution
they will be in equilibirum
so therefore increasing the conc of preservative means the conc of free preservative in the formulation is > or = to the MIC of the preservative
why does the conc of free preservatives decrease in the presence of hydrophilic polymers?(methylcellulose)
may have a chemical interaction between the preservative and the dissolved polymer
to fix that problem , we just need to increase the conc of preservative in the formulation
what are the different flavours for the 4 different taste sensations?
4 basic taste sensations: salty, sweet, bitter and sour
- a salty taste: butterscotch, apricot, peach, vanilla, wintergreen mint
- a bitter taste: cherry, mint, anise
- a sweet taste: vanilla, fruit and berry.
- a sour taste: citrus flavours, raspberry.
flavour adjuncts (methanol and chloroform) add flavour and act to desensitise the taste receptors
preferred colour to formulation; in combination with flavours, colour should match flavour (green with mint-flavoured solutions); not prerequisite for solutions
how do we prepare and manufacture these solutions?
•Simply dissolving the solutes (actives, inactives) in solvent or solvent mixture then pass through Filtration system
•Industrial scale: large mixing vessels with ports for mechanical stirrers; vessels thermostatically controlled (maintain a certain temperature); order of addition of components is fixed (product development and scale-up)
what are the types of pharmaceutical oral solutions
oral solutions
oral syrups
oral elixirs
what are features of oral solutions
•systemic absorption of the drug
into the SI then to the blood
•formulated over a broad pH range
•usual pH:~ 7.0 (unless issues regarding solubility or stability of drug)
•All components of the formulation should be soluble, with no evidence of precipitation.
what are features of oral syrups?
highly concentrated, aqueous solutions of sugar or sugar substitute; flavouring agent (cherry syrup)
unflavoured syrup: aqueous solution containing 85% sucrose.
drugs: directly incorporated into these systems or added as the syrup is being prepared.
•choice of syrup vehicle: physicochemical properties of drug. i.e. orange syrup: acidic therefore solubility of acidic drugs may decrease [precipitation of the drug].
what are the oral syrup components?
purified water
sugar(sucrose) or sugar substiutes(artificial sweeteners)(60-80%)
for syrups, already have inherent sweetness and moderately high viscosity so theres no addition of sweetening agents or viscosity modifying agents
in syrups, we have a high conc of sucrose so not a lot of available water so no microbial growth so we dont use preservatives
non sucrose bases can also be used: sorbitol solution USP(64% w/w)
sugar free syrups: for children and diabetic patients
what are the oral syrup components?
flavours:
natural origin (peppermint, herbs, lemon, spices)
synthetic flavours
some flavours have mild therapeutic activity: e.g. lots of antiacids contain mint due to its carminative properties
colours
what are oral elixirs?
clear, hydroalcoholic solution formulated for oral use
the conc of alcohol is sufficient enough to ensure that all of the other components remain in solution>10% v/v; other polyol co-solvents may be incorporated
however the presence of alcohol may be a problem for paediatric formulations or adults who wish to avoid alcohol or cant consume it
preservatives are not needed for elixirs with >12%v/v alcohol as they have enough antimicrobial properties
theophylline elixir

question:
