bonding theory + Lewis structure
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
chemical bond
involves the electrons only
nuclei of the different atoms are not affected
Quantum Theory
To be electrically neutral (# of protons = # of electrons)
To acquire the more stable electron structure of the nearest Noble Gas
orbital
region of space where electrons are most likely to be found
non-valance electrons
in the inner energy levels
valance electrons
in the outer shell
paired or single
lone pair
two electrons (pair)
repelling effect on electrons in any nearby orbitals
bonding electron
single electron
share that electron with another atom (chemical bonds with other atoms)
covalent bonding
share electrons
ionic bonding
transfer electrons
Octet Rule
obeyed by Main Group atoms
C, N, O, F atoms always obey Octet Rule when bonding
max. eight electrons can occupy orbitals in the valence energy level
Pauli Exclusion Principle
two electrons may share the same region of space
never more than two
occupy empty valence orbitals before forming electron pairs
Chemical Formulae
express the structure of atoms
identify element
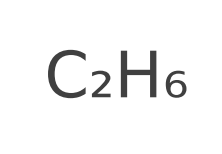
Molecular Formula
chemical formula of a chemical compound
shows the kinds and numbers of atoms in the compound
NOT REDUCED
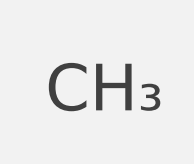
empirical formula
shows elements in a compound in their lowest whole-number ratio
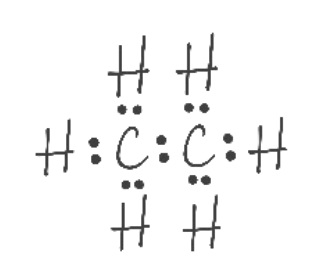
Lewis Structure
amount of valence electrons
which types of bonds are involved
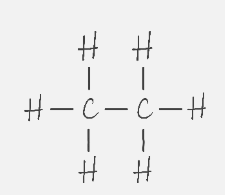
Structural Formula (Kekulé Structure)
Displays the atoms of the molecule in the order they are bonded
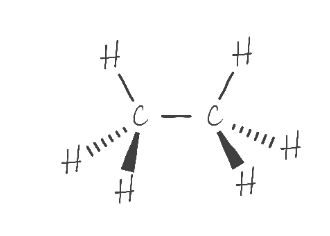
Perspective Drawing (Stereochemistry)
structure of a molecule in 3D
which atoms are above and below the plane of the paper
central atom is always assumed to be in the plane of the paper
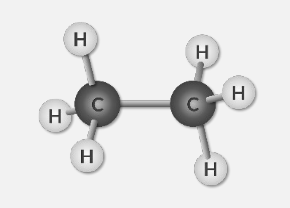
Ball and Stick Model
3D arrangement of the atoms in space
shape of the molecule
Lewis diagram
bonding electrons
lone pairs
octect rule
only valance shell
covalent bonding
rules for placing electrons
One electron dot is placed in each of the four valence orbitals before electron pairing
If there are five to eight valence electrons, a second electron dot paired with a bonding electron (single)