Chapter 3: Chemical Reactions and Reaction Stoichiometry
Stoichiometry
Stoichiometry means there are ratios between your reactants and your products.
It is an area of study that examines the quantities of substances consumed and produced in chemical reactions.
Chemical Equations
Chemical equations are how chemists represent chemical reactions on paper.
Arrows separate the starting materials (on the left), called reactants, from the ending materials (on the right), called products.
Reactants → Products
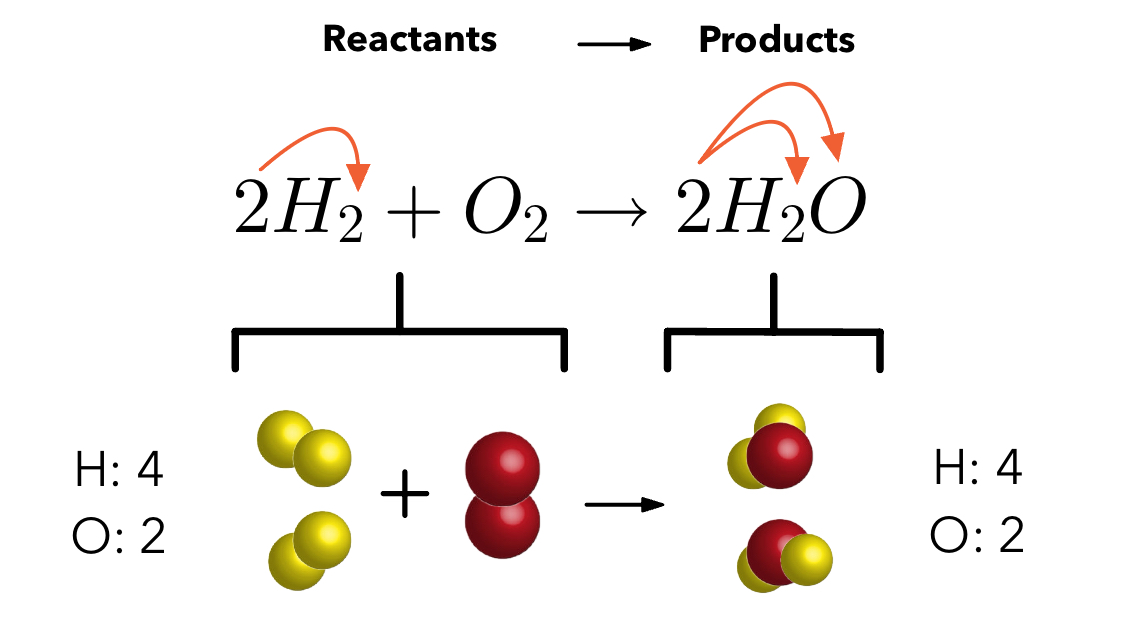
Balancing Equations
Ensure there are the same amount of elements on each side.
Do not change the coefficients.
The visual above shows there are 4 Hydrogens and 2 Oxygens on each side.
Other Symbols in Chemical Equations
The states of matter for the reactants and products are often written in parentheses to the right of each formula or symbol.
gas (g)
liquid (l)
solid (s)
aqueous (aq)
Example: CH4(g) + 2O2 (g) → CO2 (g) +2H2O (g)
Patterns of Chemical Reactivity
Combustion Reactions
In a combination reaction, two or more substances react to form one product.
A + B → C
Example of a Combination Reaction Formula: C(s) + O2(g) → CO2(g)
Decomposition Reactions
In a decomposition reaction one substance breaks down into two or more substances.
C → A + B
Example of a Decomposition Reaction: 2KClO3(s) → 2KCl(s) + 3O2(g)
Combustion Reactions
In a combustion reactions there are rapid reactions that produce a flame, they often involve oxygen in the air as a reactant.
When burning compounds with C and H in them, the products are always H2O and CO2.
Example of a Decomposition Reaction: C3H8 (g) + 5O2 (g) —> 3CO2 (g) + 4H2O (g)
Carbon dioxide
Water vapor
Formula Weight (FW)
A formula weight is the sum of the atomic weights (AW) for the atoms in a chemical formula.
For an element like sodium (Na), the formula weight is the atomic weight (23.0 amu).
The formula weight for compounds and polyatomics requires finding the atomic weight for each individual element. Multiply the element’s atomic weight by the coefficient (if there is one).
Formula Weight equation: #(AW) + #(AW) = FW
AW = Atomic Weight
FW = Formula Weight
Example: Find the formula weight of sulfuric acid H2SO4:
There are 2 Hydrogens, 1 Sulfur, and 4 Oxygens.
2(AW of H) + 1 (AW of S) + 4(AW of O) = FW
2(1.0 amu) + 32.1 amu + 4(16.0 amu)
FW of H2SO4 = 98.1 amu
Percent Composition
One can find the percentage of the mass of a compound that comes from each of the elements in the compound.
Percent Composition equation:
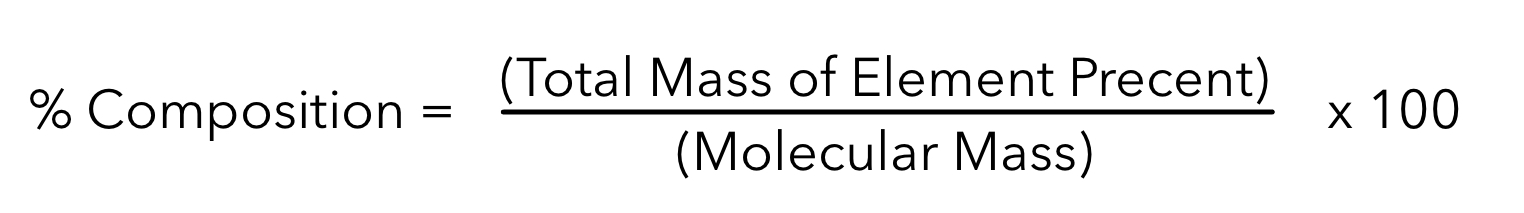
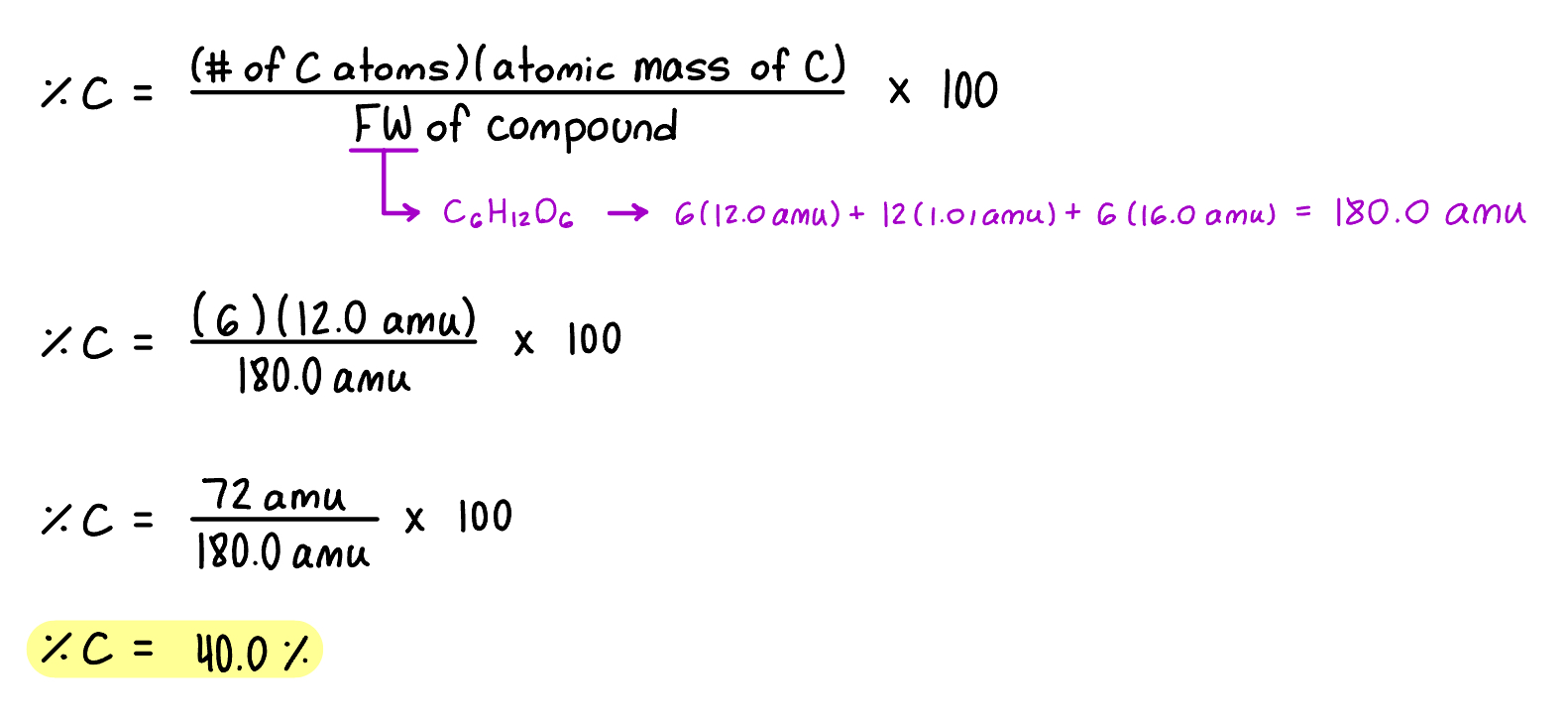
Example: Determine the percentage of carbon in glucose (C6H12O6)
Avogadro’s Number
Avogadro’s number: 6.02 × 1023 atoms or molecules is the number of particles in one mole (mol).
Molar Mass
A molar mass is the mass of 1 mol of a substance.
The molar mass of an element is the atomic weight for the element from the periodic table. If it is diatomic, it is twice that atomic weight.
The formula weight (in amu) will be the same number as the molar mass (in g/mol).
H = 1.01 g/mol
Converting Amounts
Moles provide a bridge from the molecular scale to the real-world scale.
Using equalities, we can convert from mass to atoms or from atoms to mass.
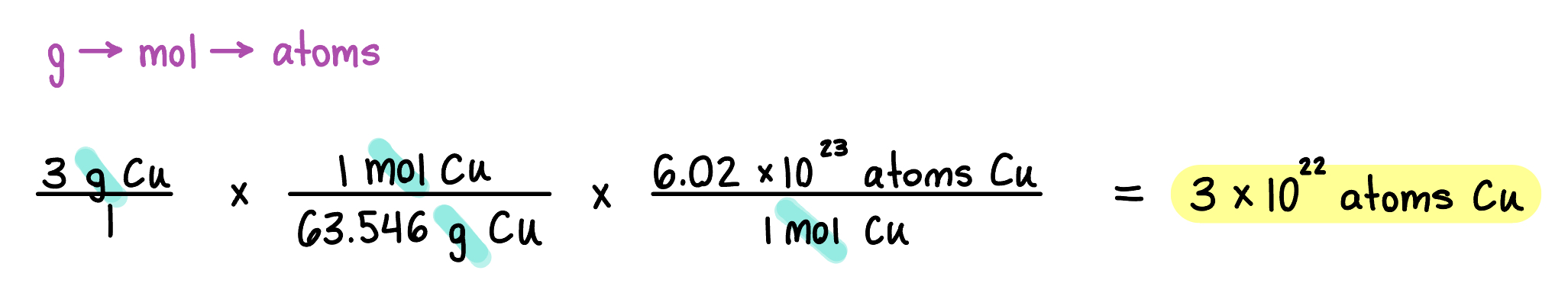
Example: Convert 3 grams of copper (Cu) into atoms
Determining Empirical Formulas
One can determine the empirical formula from the percent composition by following these three steps:
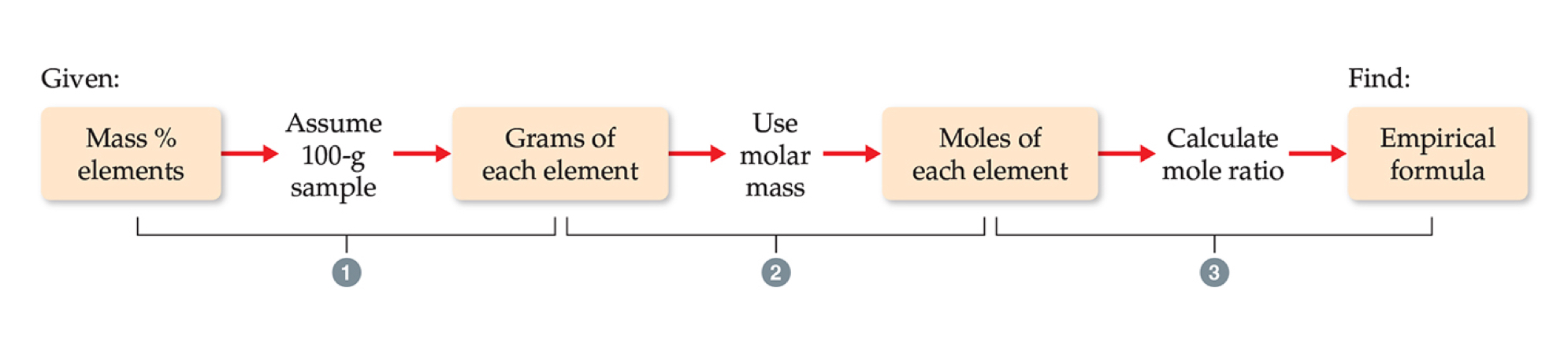
Steps for determining empirical formulas
Assume the sample is 100 grams.
Convert percentages to grams.
Covert to grams to mols.
Calculate the mole ratio by dividing by the smallest number of moles.
Use results as subscripts in empirical formula.
Example
The compound para-aminobenzoic acid is composed of carbon (61.31%), hydrogen (5.14%), nitrogen (10.21%), and oxygen (23.33%). Assuming it is a 100 g sample, find the empirical formula.
Step 1: Convert percentage to grams (assume is is a 100 gram sample).
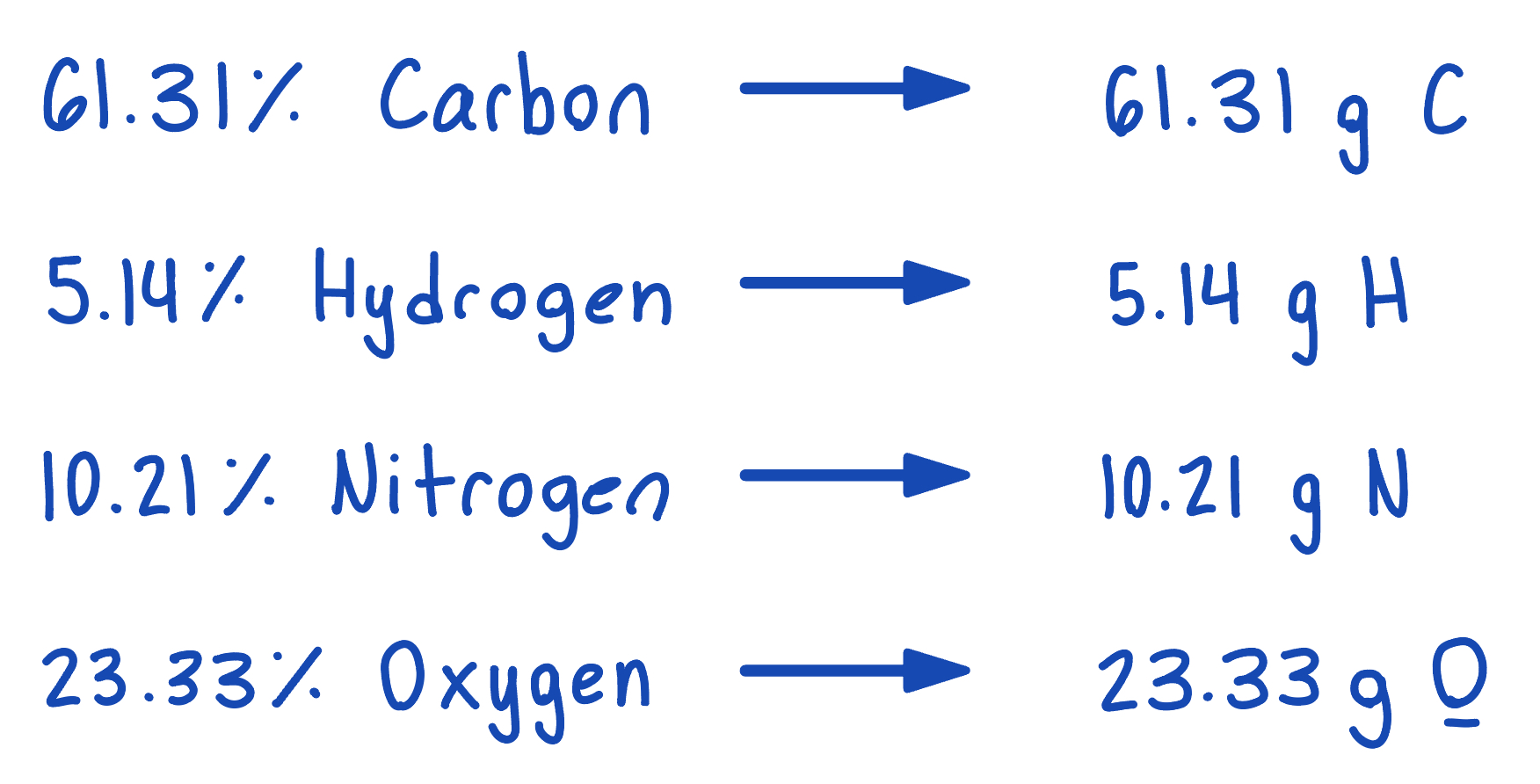
Step 2: Convert grams to mols and determine the smallest value.

Step 3: Divide mols by smallest value.

Step 4: Use results from previous step to create the empirical formula.
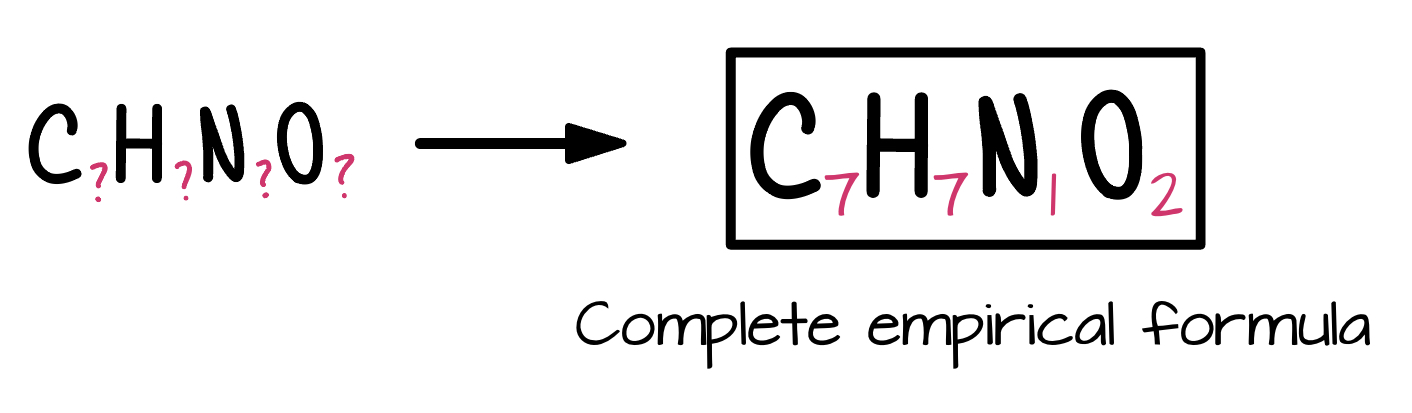
Final answer: C7H7N1O2
Determining a Molecular Formula
The number of atoms in a molecular formula is a multiple of the number of atoms in an empirical.
If we find the empirical formula and know a molar mass (molecular weight) for the compound, we can find the molecular formula.
Example: If CH has a molar mass of 78 g/mol what is the molecular formula?
CH mass = 13 g/mol
C = 12.01 g/mol
H = 1.01 g/mol
Added together: 13 g/mol
Equation: \dfrac{molar\;mass\;of\;molecular\;formula}{molar\;mass\;of\;empirical\;formula}
Ratio: \dfrac{78}{13} = 6
(CH) x 6 = C6H6
Benzene
Quantitative Information from a Balanced Equation
The coefficients in the balanced equation show:
Relative numbers of molecules of reactants and products.
Relative numbers of moles of reactants and products, which can be converted to mass.
Percent Yield
The theoretical yield is the maximum amount of product that can be made.
It is the amount of product possible as calculated through the stoichiometry problem.
The actual yield is the amount one actually produces and measures.
The percent yield can be found by comparing the amount actually obtained (actual yield) to the amount it was possible to make (theoretical yield)
Percent yield equation:

Limiting Reactants and Excess Reagents
The limiting reactant is the reactant present in the smallest stoichiometric amount.
It is the reactant you will run out of the first.
It is used in all stoichiometry calculations to determine amounts of products that are produced and amounts of any other reactant(s) that are used in a reaction.
The excess reagent is what is left over.
Reference: Chemistry The Central Science (14th Edition)