Mineralogy Test 1 (book notes)
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
What are some uses of minerals in society?
Minerals are used as resources (ores for metals, industrial materials), gems, construction materials (quartz sand, limestone), and in technology (rare earth elements in electronics).
What are silicate minerals, and why are they important?
Silicates are minerals that contain the SiO₄ tetrahedron as their fundamental building block. They make up over 90% of Earth’s crust (examples: quartz, feldspar, micas, pyroxenes).
What is the difference between descriptive and systematic mineralogy?
Descriptive mineralogy: identifying and characterizing minerals by physical/optical properties.
Systematic mineralogy: classifying minerals based on chemistry and crystal structure.
How does mineralogy relate to other sciences?
Mineralogy overlaps with chemistry (bonding, composition), physics (optics, crystallography), materials science (crystal defects, properties), and geology (Earth processes, rock classification).
What is the difference between crystalline and amorphous substances?
Crystalline: atoms arranged in a repeating, ordered pattern (quartz, halite).
Amorphous (mineraloid): no long-range atomic order (obsidian, opal).
Name the basic symmetry elements in crystals.
Rotation axes (2-fold, 3-fold, 4-fold, 6-fold).
Mirror planes (reflection symmetry).
Center of inversion (point through which all parts are reflected).
Rotoinversion axes (rotation + inversion combined).
What defines a crystal system?
The relative lengths and angles of the crystallographic axes.
What are the 14 unique 3D lattice types that describe all possible crystal structures, combining the 7 crystal systems with different lattice centering (primitive, body-centered, face-centered, base-centered) called?
Bravais lattices.
What is the characteristic external shape of a crystal, reflecting its internal symmetry and growth conditions (e.g., prismatic quartz, cubic halite) called?
Crystal habit.
What is the tendency of a mineral to break along planes of weak atomic bonding, which are parallel to certain crystallographic directions?
Cleavage
How is cleavage related to symmetry?
Cleavage planes reflect the internal symmetry of the lattice — e.g., cubic cleavage in halite comes from equal bonding strength in three perpendicular directions.
What is the shorthand system (hkl) to describe crystal planes, using reciprocals of the intercepts that a plane makes with crystallographic axes, called?
Miller index
What is it called when a chemical compound occurs in more than one crystal structure (Example: Carbon forms diamond (cubic) and graphite (hexagonal))?
Polymorphism
What is it called when two different compounds have similar crystal structures due to similarity in atomic size and charge, allowing substitution (e.g., Mg²⁺ and Fe²⁺ in olivine)?
Isomorphism
Fill in the blanks:
________ bonds: electron transfer between cation and anion (e.g., NaCl).
________ bonds: electron sharing (e.g., SiO₂ quartz).
_________ bonds: delocalized electrons across metal atoms (e.g., native copper).
_____________ bonds: weak forces between neutral atoms or molecules (e.g., graphite).
__________ bonds: moderate strength, influence layer silicates like micas.
Ionic bonds
Covalent
Metallic
Van der Waals
Hydrogen
What properties does a mineral with ionic bonds have?
It is brittle, soluble in water, high melting points.
What properties does a mineral with covalent bonds have?
Very hard, high melting points, low conductivity.
What properties does a mineral with Van der Waals/Hydrogen-bonds have?
Soft, easy cleavage, low melting points.
What is a cation and an anion?
Cation: positively charged ion (lost electrons).
Anion: negatively charged ion (gained electrons).
What is the effective size of an ion called and what does it do for crystal structures?
The ionic radius; it determines how ions pack together in crystals, affecting coordination number, geometry, and stability of the structure
What is the number of nearest-neighbor ions of opposite charge surrounding a central ion in a crystal structure called?
The coordination number.
What is Pauling’s first rule?
Coordination principle- A cation’s coordination number is determined by the ratio of cation to anion radii, which sets the ideal geometric arrangement of anions around the cation.
What is Pauling’s second rule?
Electrostatic valency principle- The strength of the electrostatic bond to a central ion equals the charge of the ion divided by its coordination number. The sum of bond strengths to an anion should equal the anion’s charge.
What is Pauling’s third rule?
Sharing of polyhedral elements- The stability of a structure decreases when polyhedra share edges or faces, especially those of cations with high charge and small size.
What is Pauling’s fourth rule?
Cations of high valence avoid adjacency- Large, highly charged cations tend to stay apart to maximize crystal stability and reduce electrostatic repulsion.
What is Pauling’s fifth rule?
Principle of parsimony- The number of different types of constituents in a crystal tends to be small. Crystals favor simple, repeatable structures.
What factors control ionic substitution in minerals?
Size similarity: ionic radii must be within ~15%.
Charge balance: substitutions must maintain neutrality.
Crystal structure tolerance: lattice must accommodate substituted ions.
What is the range of compositions in a crystal structure formed by substitution of one ion for another, forming a continuous chemical series called?
Example: (Mg,Fe)₂SiO₄ in olivine (forsterite ↔ fayalite)
Solid Solution
What is the difference between complete and partial solid solutions?
Complete: ions can substitute freely across the entire compositional range.
Partial: substitution is limited by size, charge, or structure constraints.
What is the significance of cation-anion charge balance in minerals?
Charge balance ensures electrical neutrality. Substitutions must preserve total charge, influencing composition and stability.
How does crystal structure control mineral properties?
Atomic arrangement affects density, hardness, cleavage, optical properties, and reactivity.
Example: Tetrahedral SiO₄ units in quartz → hard, durable, no cleavage.
Fill in the blanks:
_________ site: cation surrounded by 4 anions in a tetrahedron.
_________ site: cation surrounded by 6 anions in an octahedron.
Tetrahedral
Octahedral
Describe crystal field effects in transition metals.
The arrangement of surrounding anions affects the splitting of d-orbitals in transition metal ions, influencing color, magnetic properties, and stability.
What are common examples of ionic substitution in minerals?
Plagioclase feldspars: Na⁺ ↔ Ca²⁺
Olivine: Mg²⁺ ↔ Fe²⁺
Pyroxenes: Ca²⁺, Na⁺ substitutions
How do pressure and temperature affect crystal chemistry?
They can change coordination numbers, stabilize different polymorphs, and influence ionic substitution.
Example: High pressure favors denser coordination (octahedral → higher CN).
How do polyhedral connections affect crystal stability?
Corner-sharing polyhedra are more stable than edge- or face-sharing due to reduced cation-cation repulsion.
The three components of a crystal structure:
1. __________: An array of points representing the repeating pattern.
2. __________: The atom(s) attached to each lattice point.
3. __________: The smallest repeating unit that represents the entire lattice.
lattice
motif
unit cell
Name the 7 crystal systems.
Cubic (P, I, F), Tetragonal (P, I), Orthorhombic (P, C, I, F), Monoclinic (P, C), Triclinic (P), Hexagonal (P), Rhombohedral (R)
Describe the isometric crystal system.
Isometric (unit cell like a cube): all sides are of equal length and angles between sides are 90 degrees
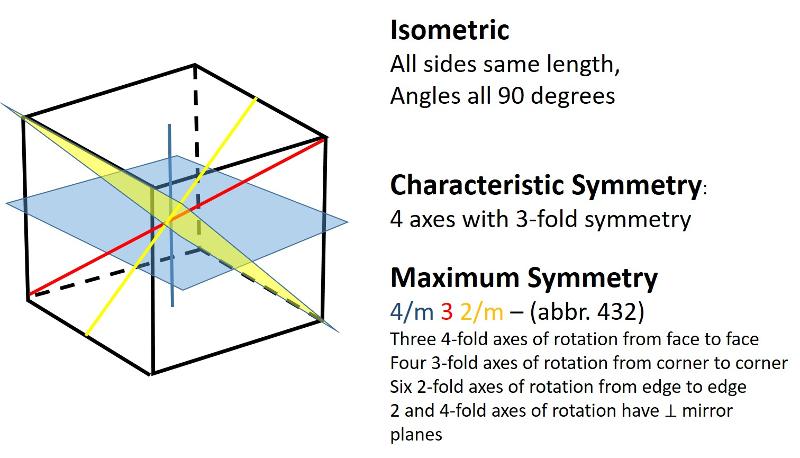
Describe the tetragonal crystal system.
Tetragonal (unit cell like an elongated or shortened cube): One side has a different length from the other two sides which are equal. Angles between sides are again 90 degrees.
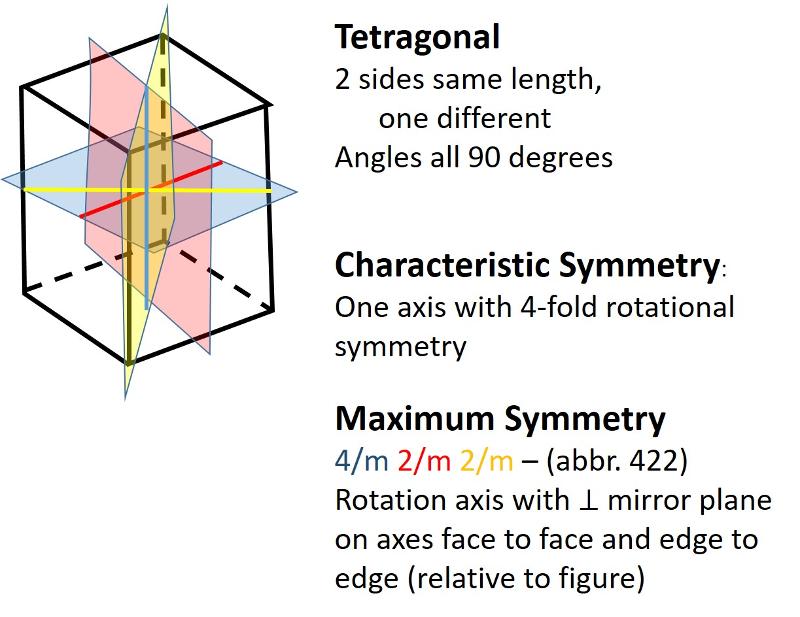
Describe the orthorhombic crystal system.
Orthorhombic (unit cell like a cube elongated or shortened in 2 dimensions): All sides are different lengths. Angles between sides are again 90 degrees.
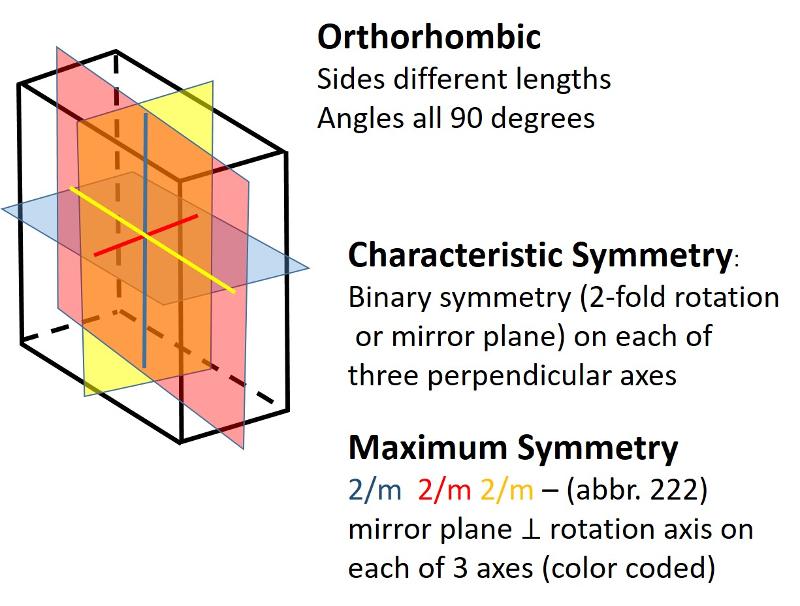
Describe the monoclinic crystal system.
Monoclinic (unit cell like a cube sheared-over in one direction): All sides can be different lengths. Of the 3 non-parallel sides, the angle between one pair is not 90 degrees, the other two pairs are at 90 degrees.
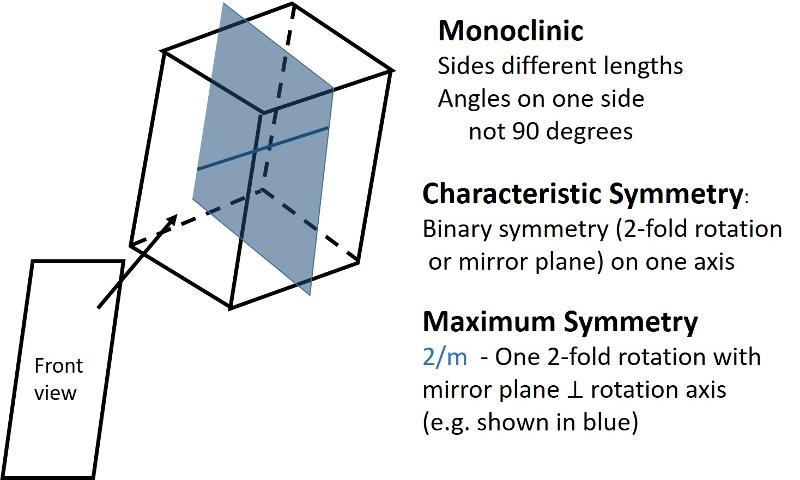
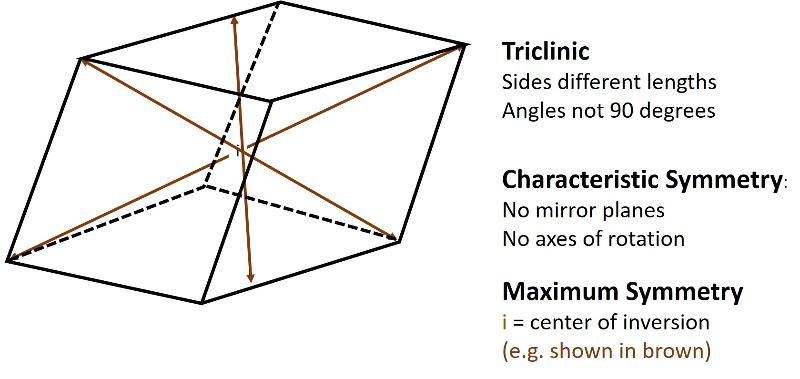
Describe the triclinic crystal system.
Triclinic (unit cell like a cube sheared over in three-directions). All sides can be different lengths. The angles between the 3 non-parallel sides differ from 90 degrees.
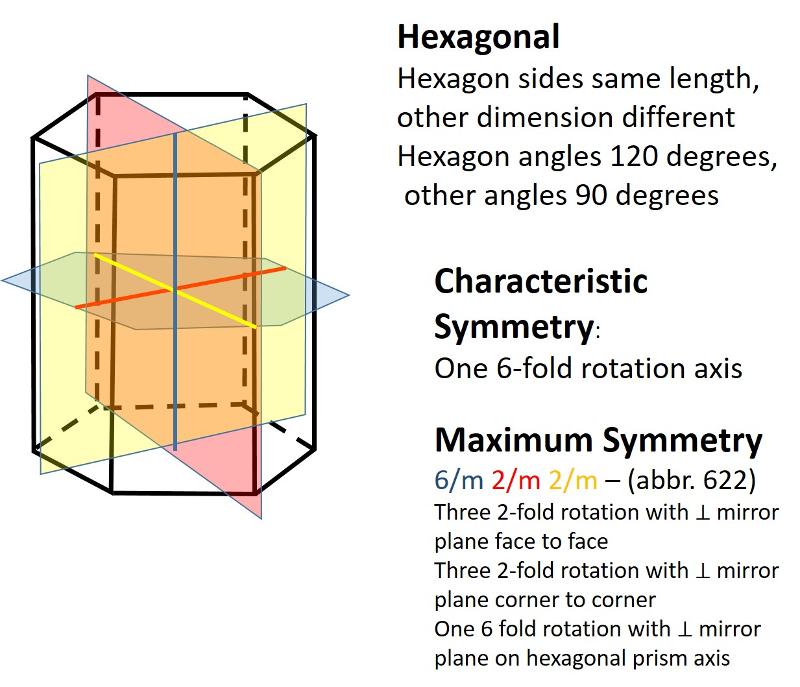
Describe the hexagonal crystal system.
Hexagonal (unit cell like a one-third portion of a hexagonal prism), Length of one axis differs from others, with angles of 120 degrees and 90 degrees)
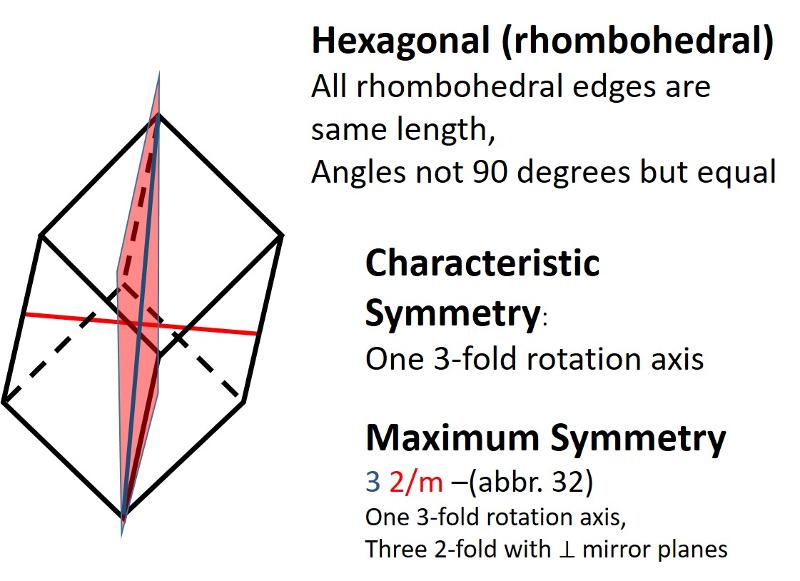
Describe the rhombohedral crystal system.
Rhombohedral (a subset of Hexagonal, but with 3-fold rotation instead of 6-fold, all sides are equal length and all angles differ from 90 degrees)
What is the difference between a primitive (P) and centered lattice?
Primitive (P): lattice points only at the corners of the unit cell.
Centered: additional lattice points inside the cell (e.g., face-centered F, body-centered I, base-centered C).
What is a crystal structure’s packing efficiency?
The fraction of volume in a unit cell actually occupied by atoms. High packing efficiency indicates tightly packed atoms.
Example: FCC cubic = 74%
Example: BCC cubic = 68%
What is the difference between primitive cubic, body-centered cubic, and face-centered cubic?
Primitive cubic (P): lattice points at corners only, low packing efficiency (~52%).
Body-centered cubic (BCC): corners + one center point, packing ~68%.
Face-centered cubic (FCC): corners + face centers, packing ~74%.
What are the different stacking arrangements of identical layers in a crystal, often seen in layer silicates and carbon polymorphs (graphite vs. hexagonal diamond)?
Polytypes
Crystal __________: lines connecting lattice points, represented as [uvw] in Miller indices.
Crystal ________: flat surfaces within the lattice, represented as (hkl) in Miller indices.
directions
planes
What is anisotropy in crystals?
Direction-dependent physical properties (e.g., optical behavior, hardness, conductivity). Caused by differences in atomic arrangement along different axes.
What is the difference between crystal symmetry and crystal habit?
Symmetry: arrangement of points and atoms that repeat in space.
Habit: external shape that reflects internal crystal symmetry (e.g., cubic halite, hexagonal quartz).
What is a defect in crystal structure?
Imperfections in the ideal lattice,
________ defects: vacancies, interstitials, substitutions
________ defects: dislocations
________ defects: stacking faults, grain boundaries
point
line
planar
What are minerals with the same chemical composition but different crystal structures due to differences in pressure, temperature, or formation conditions called?
Example: Calcite vs. Aragonite (CaCO₃)
Polymorphs
How do stacking sequences affect crystal structure?
The arrangement of atomic layers affects symmetry, unit cell dimensions, and polymorphic forms (e.g., hexagonal vs. cubic close packing).
What is a geometrical figure formed by a cation surrounded by its anions that determines bonding, density, and stability of the mineral structure?
Coordination polyhedra.
What are the main stages of crystal formation?
Nucleation: Formation of a small, stable cluster of atoms or ions.
Crystal growth: Addition of atoms or ions to the existing nucleus to form larger crystals.
What is the initial step in crystal formation where a critical-size cluster of atoms or ions forms and becomes a stable nucleus?
Nucleation
What is the difference between homogeneous and heterogeneous nucleation?
Homogeneous nucleation: Occurs uniformly throughout the solution or melt without foreign surfaces. Requires greater supersaturation.
Heterogeneous nucleation: Occurs on pre-existing surfaces (e.g., container walls, impurities). Requires lower supersaturation.
What is it called when a solution contains more solute than it can normally dissolve at equilibrium, driving nucleation and crystal growth?
Supersaturation
Factors controlling crystal growth rate:
________ level: higher level → faster growth
________: affects diffusion rates
Presence of _________: can inhibit growth
Solution _________: availability of growth ions
Supersaturation
Temperature
Impurities
Composition
What is a branching, tree-like crystal growth pattern that occurs under rapid growth conditions and high supersaturation?
Dendritic growth
What is euhedral, subhedral, and anhedral crystal growth?
Euhedral: well-formed crystal faces, fully developed
Subhedral: partially developed faces
Anhedral: no crystal faces, irregular shape
How does solution temperature affect crystal growth?
Higher temperatures generally increase ion mobility and growth rates, but may also decrease supersaturation if solubility increases.
How does pressure influence crystal growth?
High pressure can stabilize certain crystal structures, affect diffusion rates, and influence nucleation in melts and fluids.
What is interface-controlled growth?
Growth controlled by the rate at which atoms attach to the crystal surface. Occurs when ion diffusion is fast compared to attachment.
What is diffusion-controlled growth?
Growth controlled by the rate at which ions or atoms diffuse through the surrounding medium to reach the crystal surface.
How do impurities affect crystal growth?
Can slow or inhibit growth by blocking attachment sites
Can lead to irregular or distorted crystal shapes
Can promote zoning if incorporated unevenly
What is the variation in composition within a single crystal due to changes in growth conditions (temperature, pressure, chemistry), visible in layers?
Crystal zoning.
Magma composition influencing crystal growth:
______ magmas → slow growth, well-formed crystals
______ magmas → rapid growth, often smaller, less euhedral crystals
Silica rich
Mafic
What are some crystal habit modifiers?
Factors like temperature, supersaturation, impurities, and solvent conditions affect the external shape (habit) of a crystal.
How do fluid inclusions form during crystal growth?
Tiny pockets of fluid are trapped within a growing crystal, recording conditions (temperature, pressure, chemistry) at the time of growth.
Crystal Growth:
_________ growth: growth during initial formation (e.g., from magma or solution).
_________ growth : growth during later alteration, metamorphism, or recrystallization.
Primary
Secondary
Why is color often unreliable for mineral identification?
Because the same mineral can have different colors due to impurities, defects, or weathering (e.g., quartz can be clear, pink, purple, or gray).
What is luster?
Luster is the way a mineral reflects light from its surface. Types include:
Metallic: shiny like metal (e.g., pyrite)
Nonmetallic: includes vitreous (glassy), resinous, pearly, silky, greasy, or dull
Main types of luster:
________: glassy, shiny (e.g., quartz)
________: looking like resin or amber (e.g., sphalerite)
________: iridescent, like pearls (e.g., muscovite)
______: fibrous sheen (e.g., asbestos)
_______: appears oily (e.g., nepheline)
________ no shine, rough texture (e.g., kaolinite)
Vitreous
Resinous
Pearly
Silky
Greasy
Dull/Earthy
How can surface quality/type affect luster?
Smooth, well-formed crystal faces reflect light more effectively: shiny luster.
Rough or irregular surfaces: dull luster.
Main types of crystallographic techniques?
1. ______________ – determines atomic arrangements.
2. ____________ – uses electrons to analyze crystal structure, often in TEM.
3. _____________ – similar to XRD but uses neutrons; good for locating light elements like H.
X-ray diffraction (XRD
Electron diffraction (ED)
Neutron diffraction
What is a technique that uses X-rays to probe the spacing of atomic planes in a crystal?
X-ray diffraction
What is the difference between powder XRD and single-crystal XRD?
Powder XRD: Uses powdered mineral; produces a diffraction pattern of many crystals; good for phase identification.
Single-crystal XRD: Uses a single crystal; provides detailed 3D atomic positions and accurate bond lengths/angles.
Main components of an XRD instrument:
1. X-ray source – _________________
2. Goniometer – ______________________
3. Sample holder – _________________
4. Detector – ___________________________
produces X-rays (commonly Cu Kα).
positions sample and detector.
supports single crystal or powder.
measures intensity and angle of diffracted X-rays.
What is a plot of X-ray intensity vs. diffraction angle (2θ) called?
Diffractogram
What do the peaks in a diffractogram correspond to?
Peaks correspond to specific interplanar spacings, which can be used to identify minerals and determine unit cell parameters.
How is crystallinity assessed?
_______ peaks → high crystallinity
_______ peaks → poor crystallinity or amorphous material
By analyzing XRD peak sharpness and intensity.
sharp
broad
What are common errors or limitations in crystallographic techniques?
Sample impurities or defects can distort peaks.
Preferred orientation can affect intensity in powders.
Small crystals may produce weak or broadened peaks.
Overlapping peaks in complex mixtures can complicate analysis.
What is the general workflow for converting a chemical analysis to a formula?
1. Convert weight % oxides → moles of cations
2. Multiply by oxygen atoms per oxide → moles of oxygen
3. Sum all oxygens → total oxygen
4. Normalize cations to match mineral’s oxygen count
5. Assign cations to crystallographic sites
6. Verify charge balance and structural plausibility
___________ formula: calculated directly from analysis, normalized to a fixed number of anions.
___________ formula: reflects perfect stoichiometry and site occupancy based on crystal chemistry rules.
Empirical
Ideal
How do substitutions in minerals work?
Some elements can replace others in the crystal structure. Include them in the site they prefer, keeping charge balance.