Antibacterials & Antifungals & Emerging therapeutics
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What are the types of antibacterial medication?
1. Small molecule drugs E.g. Antibiotics
2. Antibacterial peptides
3. Bacteriophage
What are antimicrobial peptides, and what is their primary source?
Antimicrobial peptides are short chains of amino acids that kill bacteria by disrupting their cell membranes
They are sourced naturally from humans (146), animals (2246), plants (346), and synthetically (1783)."
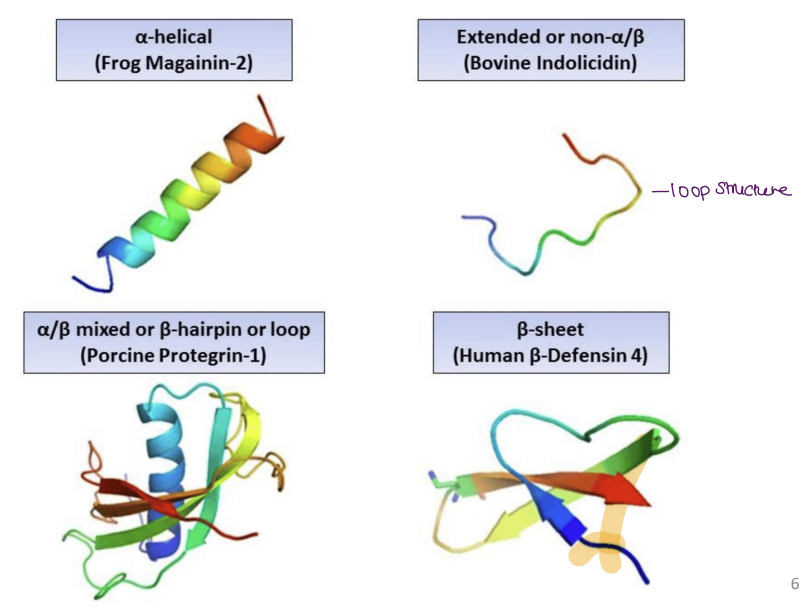
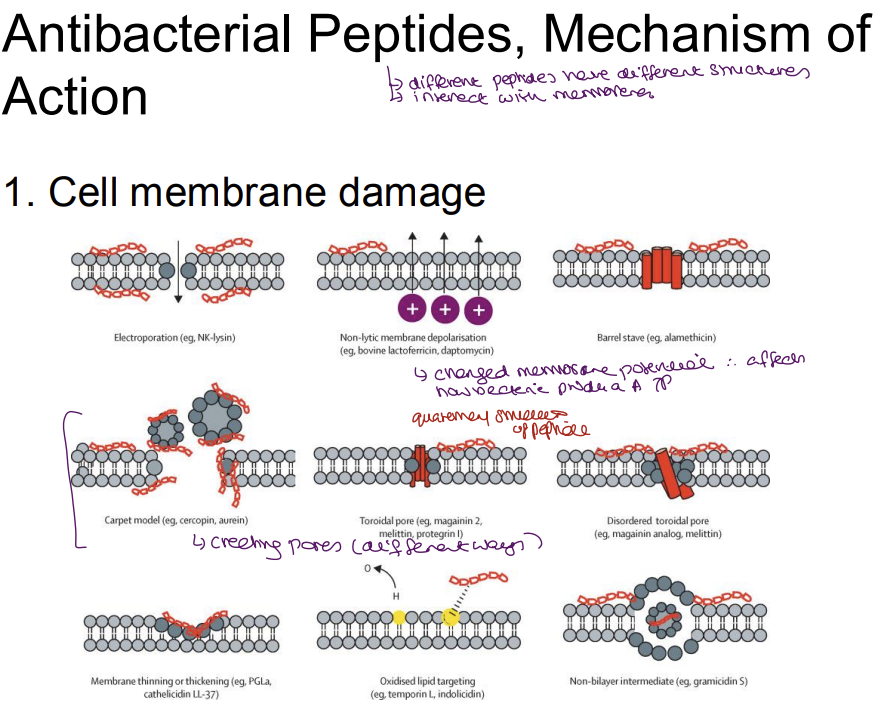
Describe the mechanism of action for antimicrobial peptides
1. Membrane Disruption (Primary Mechanism)
Bacterial membranes (negatively charged due to lipopolysaccharides/teichoic acids) are damaged by Positively charged AMPs which bind to negative bacterial membranes → disrupt lipid bilayer.
Electroporation: Create pores → ion leakage → cell death.
Carpet model: AMPs coat membrane → solubilize it.
Selectivity: Human membranes (neutral phospholipids) remain unharmed.
2. Intracellular Damage
Ribosomes: Inhibit protein synthesis (e.g., bind to 30S/50S subunits).
DNA/RNA: Interfere with replication/transcription (e.g., peptide-nucleic acid interactions).
Proline-rich AMPs (e.g., pyrrhocoricin) enter bacteria → bind heat shock proteins → disrupt proteostasis.
3. Immune Modulation
Pro-inflammatory Effects:
Induce cytokine release (e.g., IL-1β, TNF-α) → recruit macrophages/neutrophils.
Activate TLRs (e.g., TLR4) → enhance pathogen clearance.
Anti-inflammatory Effects:
Some AMPs (e.g., LL-37) resolve excessive inflammation.
4. Additional Actions
Biofilm disruption: Degrade bacterial extracellular matrix.
Synergy: Enhance conventional antibiotics (e.g., break resistance).
Describe the advantages and disadvantages of antimicrobial peptides
Advantages
Target Specificity & Safety
3D Structure: AMPs bind to bacteria-specific targets (e.g., anionic [+ve] membranes, unique protein amino acid sequences) → minimal harm to human cells.
Lower Adverse Effects: Unlike broad-spectrum antibiotics, AMPs spare the microbiome and reduce collateral damage.
Immune Modulation
Enhance Host Defenses: Some AMPs (e.g., LL-37, defensins) stimulate cytokine release (e.g., IL-1β, TNF-α) → recruit immune cells (macrophages, neutrophils).
Anti-Endotoxin Effects: Neutralize bacterial LPS, reducing septic shock risk.
Low Resistance Risk
Multi-Mechanistic Action [Broad Bacterial Inhibition]: Simultaneously disrupt membranes, inhibit ribosomes, and damage DNA → bacteria struggle to evolve resistance.
Daptomycin (cyclic lipopeptide) remains effective against MRSA.
—
Challenges
Formulation Difficulties
Oral Bioavailability: Large size (~2–10 kDa) and hydrophilicity limit absorption → mostly restricted to IV/topical routes.
Solutions: Nanoparticle encapsulation, PEGylation, or prodrug designs.
Stability Issues
Protease Degradation: Susceptible to stomach acids (pepsin) and serum proteases → short half-life.
Workarounds: Non-natural amino acids (e.g., D-enantiomers), cyclization, or peptidomimetics.
High Production Costs
Complex Synthesis: Solid-phase peptide synthesis (SPPS) is expensive at scale.
Purification Hurdles: HPLC purification adds cost vs. small-molecule drugs.
What are bacteriophages, and how are they used therapeutically?
Bacteriophages are viruses that infect and destroy bacteria
They are used therapeutically to target specific bacterial infections by injecting their genetic material into bacteria, leading to bacterial lysis.
—
Precision Weapons: Target-specific, leaving human cells unharmed.
Dual Action: Lytic phages kill instantly / Lysogenic phages can hide and spread genes.
Medical Potential: Used in therapy, diagnostics, and even CRISPR delivery.
Describe what each bacteriophage component is for
capsid head- contains phage ds DNA
tail sheath- DNA travel from head to sheath when infecting
tail fibre- attaches phage to the cell
spike- protein recognises the right species, it penetration into the host and injects its DNA
1. Capsid Head: Protects the double-stranded DNA (dsDNA) genome encoding viral enzymes/replication proteins.
Icosahedral [Hexagonal] protein shell that protects genetic material.
2. Tail Sheath: Acts as a channel for DNA ejection during infection.
Contracts during infection to drive DNA ejection into the host.
3. Tail Fibers: Recognize and attach to specific receptors on the bacterial surface.
Specificity: Determines host range (e.g., T4 phage targets E. coli's LPS).
4. Baseplate Spike: Anchors the phage to the bacterial surface; triggers sheath contraction.
Dual Role:
Recognition: Binds to bacterial surface markers (e.g., proteins, sugars).
Penetration: Pierces the cell wall/membrane to inject viral DNA. (e.g., T4’s lysozyme-like enzyme degrades peptidoglycan).
Key Phage Examples
Phage | Structure | Host | Unique Trait |
|---|---|---|---|
T4 | Icosahedral head + tail | E. coli | Complex baseplate with 6 fibers |
M13 | Filamentous | E. coli | No lysis; secreted progeny |
T7 | Short tail | E. coli | Rapid lytic cycle (~17 min) |
P1 | Icosahedral + tail | E. coli | Lysogenizes as a plasmid |
![<p>capsid head- contains phage ds DNA</p><p>tail sheath- DNA travel from head to sheath when infecting</p><p>tail fibre- attaches phage to the cell</p><p>spike- protein recognises the right species, it penetration into the host and injects its DNA </p><p><strong>1. Capsid Head: </strong>Protects the <strong>double-stranded DNA (dsDNA)</strong> genome encoding viral enzymes/replication proteins.</p><ul><li><p class="ds-markdown-paragraph">Icosahedral [Hexagonal] protein shell that protects genetic material.</p></li></ul><p><strong>2. Tail Sheath: </strong>Acts as a channel for DNA ejection during infection.</p><ul><li><p class="ds-markdown-paragraph">Contracts during infection to drive DNA ejection into the host.</p></li></ul><p><strong>3. Tail Fibers: </strong>Recognize and attach to specific receptors on the bacterial surface.</p><ul><li><p class="ds-markdown-paragraph"><strong>Specificity</strong>: Determines host range (e.g., T4 phage targets <em>E. coli</em>'s LPS).</p></li></ul><p><strong>4. Baseplate Spike: </strong>Anchors the phage to the bacterial surface; triggers sheath contraction.</p><ul><li><p class="ds-markdown-paragraph"><strong>Dual Role</strong>:</p><ol><li><p class="ds-markdown-paragraph"><strong>Recognition</strong>: Binds to bacterial surface markers (e.g., proteins, sugars).</p></li><li><p class="ds-markdown-paragraph"><strong>Penetration</strong>: Pierces the cell wall/membrane to inject viral DNA. (e.g., T4’s lysozyme-like enzyme degrades peptidoglycan).</p></li></ol></li></ul><p class="ds-markdown-paragraph"></p><p><strong>Key Phage Examples</strong></p><table style="min-width: 100px"><colgroup><col style="min-width: 25px"><col style="min-width: 25px"><col style="min-width: 25px"><col style="min-width: 25px"></colgroup><tbody><tr><th colspan="1" rowspan="1" style=" padding: 10px 10px 10px 0px; border-bottom: 1px solid rgb(139, 139, 139); border-top: none; font-weight: 600; line-height: 1.72; border-right- border-left- text-align: left;"><p><strong>Phage</strong></p></th><th colspan="1" rowspan="1" style=" padding: 10px; border-bottom: 1px solid rgb(139, 139, 139); border-top: none; font-weight: 600; line-height: 1.72; border-right- border-left- text-align: left;"><p><strong>Structure</strong></p></th><th colspan="1" rowspan="1" style=" padding: 10px; border-bottom: 1px solid rgb(139, 139, 139); border-top: none; font-weight: 600; line-height: 1.72; border-right- border-left- text-align: left;"><p><strong>Host</strong></p></th><th colspan="1" rowspan="1" style=" padding: 10px; border-bottom: 1px solid rgb(139, 139, 139); border-top: none; font-weight: 600; line-height: 1.72; border-right- border-left- text-align: left;"><p><strong>Unique Trait</strong></p></th></tr><tr><td colspan="1" rowspan="1" style="padding: 10px 10px 10px 0px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><strong>T4</strong></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Icosahedral head + tail</p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><em>E. coli</em></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Complex baseplate with 6 fibers</p></td></tr><tr><td colspan="1" rowspan="1" style="padding: 10px 10px 10px 0px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><strong>M13</strong></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Filamentous</p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><em>E. coli</em></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>No lysis; secreted progeny</p></td></tr><tr><td colspan="1" rowspan="1" style="padding: 10px 10px 10px 0px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><strong>T7</strong></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Short tail</p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><em>E. coli</em></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Rapid lytic cycle (~17 min)</p></td></tr><tr><td colspan="1" rowspan="1" style="padding: 10px 10px 10px 0px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><strong>P1</strong></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Icosahedral + tail</p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p><em>E. coli</em></p></td><td colspan="1" rowspan="1" style="padding: 10px; border-bottom: 1px solid rgb(82, 82, 82); line-height: 1.72; border-top- border-right- border-left- min-width: 100px; max-width: max(30vw, 320px);"><p>Lysogenizes as a plasmid</p></td></tr></tbody></table><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b308f48c-e596-4f02-8ebd-a3cd5b94bb1d.png)
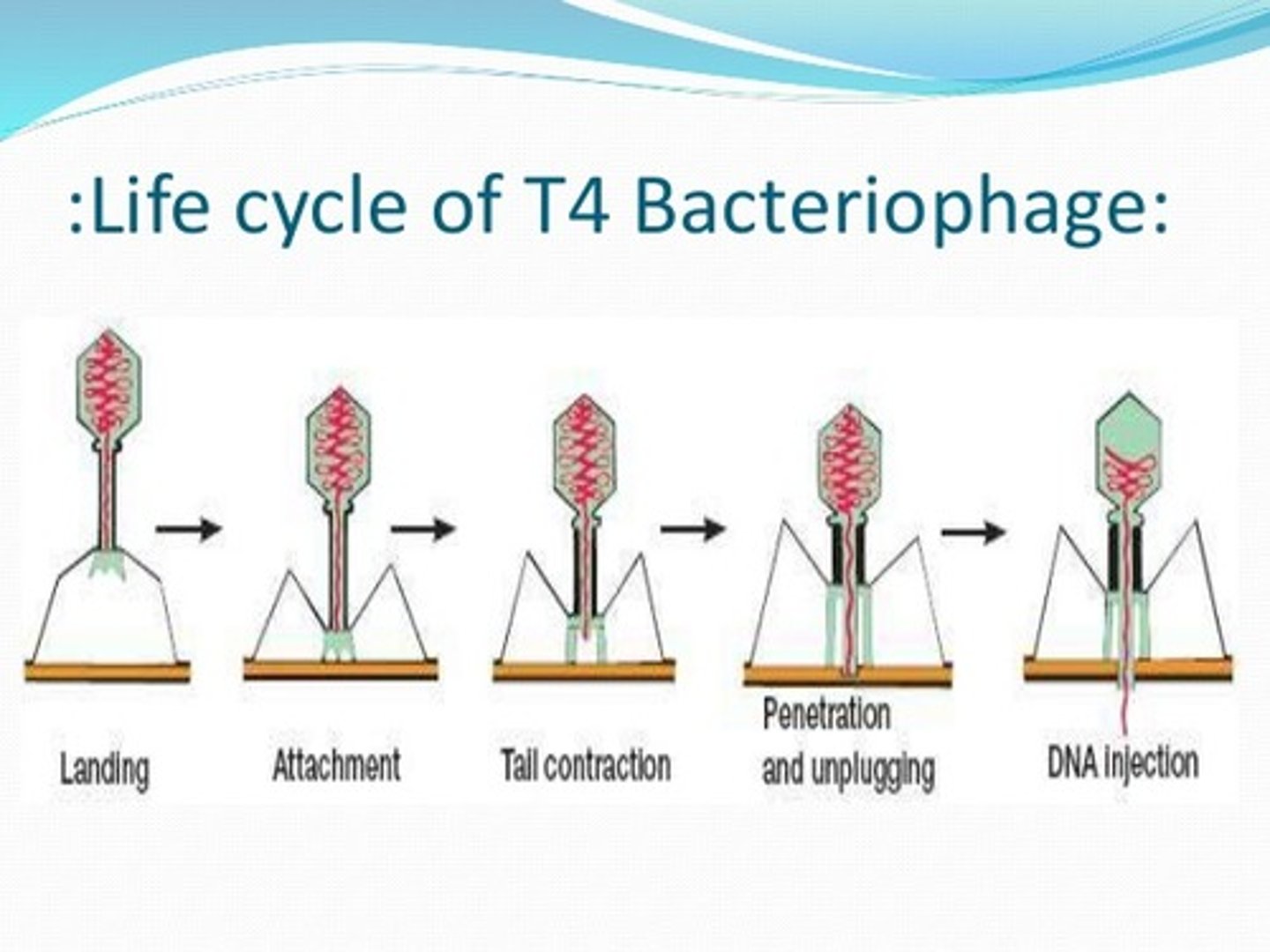
Describe the injection process
1. Host Recognition & Initial Attachment
Tail Fiber Binding:
The long tail fibers (LTFs) of T4 recognize and weakly bind to specific receptors on the E. coli surface (e.g., lipopolysaccharides or outer membrane proteins).
Reversible: Initial attachment is flexible, allowing the phage to "scan" for optimal infection sites.
2. Irreversible Adhesion
Baseplate Transformation:
Upon LTF engagement, the baseplate undergoes a dramatic conformational change—from a "dome" to a star-shaped structure.
Short tail fibers (STFs) unfold and bind irreversibly to the host, locking the phage in place.
3. Sheath Contraction & Membrane Penetration
Tail Sheath Dynamics:
The contractile sheath (made of gp18 subunits) shortens by ~50%, driving the rigid inner tail tube through the bacterial envelope.
Mechanical Force: This action generates enough pressure to pierce the outer membrane, peptidoglycan layer, and inner membrane.
4. DNA Injection
Translocation Process:
The tail tube acts as a molecular syringe, injecting viral dsDNA into the host cytoplasm.
Lysozyme Activity: The T4 spike protein (gp5) locally degrades peptidoglycan, easing DNA entry.
Speed: DNA ejection occurs in <1 second, with the genome entering the cell head-first (the terminal genes enter last).
Replication: Phage DNA hijacks host machinery to produce new virions.
Lytic: Immediate lysis (e.g., T4).
Lysogenic: DNA integrates as prophage (e.g., lambda).
What are the key differences between bacterial and fungal infections?
Treatment Strategies
Bacterial Infections:
Antibiotics: Target prokaryote-specific structures (e.g., β-lactams inhibit peptidoglycan synthesis).
Example Drugs: Penicillin, ciprofloxacin, vancomycin.
Fungal Infections:
Antifungals: Exploit eukaryotic features (e.g., azoles block ergosterol synthesis).
Example Drugs: Fluconazole, amphotericin B, caspofungin.
Feature | Bacteria (Prokaryotes) | Fungi (Eukaryotes) |
|---|---|---|
Cell Wall | Peptidoglycan (target for antibiotics like penicillin) | Chitin + glucans (target for antifungals like echinocandins) |
Cell Membrane | No sterols (except Mycoplasma) | Ergosterol (target for azoles, polyenes) |
Genome | Circular DNA, no nucleus | Linear DNA in nucleus + mitochondria |
Resistance Mechanisms:
Bacteria: Horizontal gene transfer (plasmids).
Fungi: Efflux pumps, biofilm formation.
Clinical Challenges
Bacteria: Rapid replication → acute infections (e.g., E. coli UTIs).
Fungi: Slow growth → chronic infections (e.g., Candida thrush).
What is the difference between lytic and lysogenic bacteria
Lytic bacteriophage: DNA is NOT integrated into the host's DNA Lysogenic bacteria: DNA IS integrated into the host's DNA
What are limitations of bacteriophage therapy
1. Targeted Delivery Limitations: Phages must reach the infection site, but deep-seated or systemic infections (e.g., endocarditis, biofilms) may be inaccessible.
Solution:
IV administration: Limited by rapid immune clearance (e.g., Kupffer cells in the liver).
Bioengineered carriers: Nanoparticles or hydrogels to protect phages en route.
2. Narrow Host Specificity: Phages often target a single bacterial strain (e.g., E. coli phage T4 won’t kill Pseudomonas).
Solution:
Phage cocktails: Mix multiple phages to broaden coverage (e.g., targeting Staphylococcus aureus and P. aeruginosa simultaneously).
Engineered phages: Modify tail fibers to expand host range.
3. Bacterial Resistance Risks: Receptor mutations (e.g., LPS modifications to block phage attachment). / CRISPR-Cas systems (bacterial "immune" defense against phage DNA).
Mitigation:
Phage-antibiotic synergy: Combine with antibiotics to reduce resistance (e.g., phages + ciprofloxacin).
Evolution-guided design: Pre-select phages that counter common resistance pathways.
4. High Cost & Personalization
Challenges:
Customization: Requires isolating patient-specific phages (time/lab-intensive).
Production: Complex purification (e.g., cesium chloride gradient centrifugation).
Advances:
Synthetic biology: Standardized phage "platforms" for rapid scaling.
Banking: Pre-made libraries for common pathogens (e.g., Mycobacterium abscessus phages).
Challenge | Phage Therapy | Antibiotics |
|---|---|---|
Delivery | Limited by anatomy/immune clearance | Systemic distribution |
Specificity | Strain-specific (narrow) | Broad-spectrum (collateral damage) |
Resistance | Evolves but can adapt phages | Widespread (e.g., MRSA, ESBL) |
Cost | High (personalized) | Low (mass-produced) |
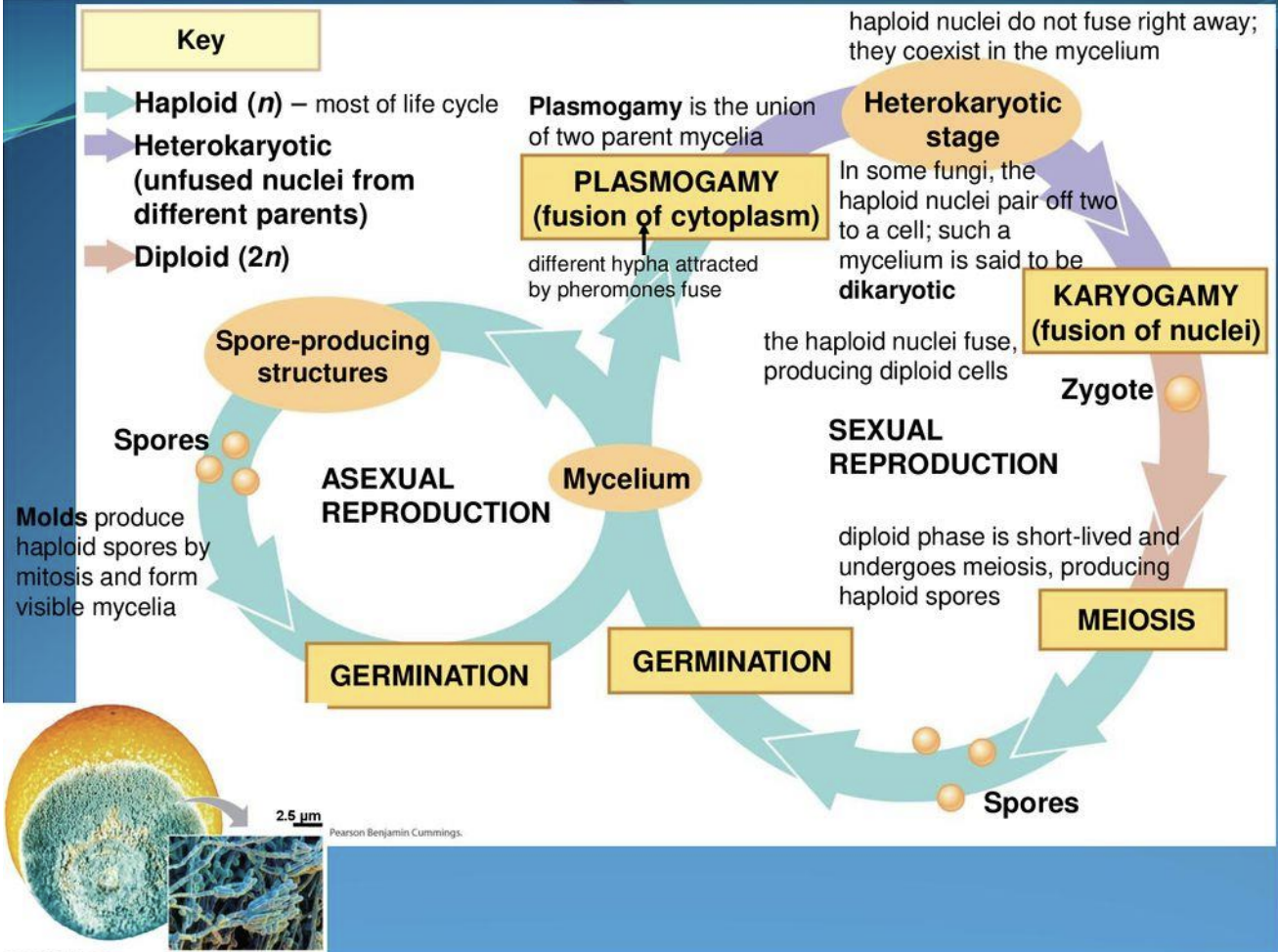
Describe fungi life cycle
2 Methods:
Sexual reproduction:
Mycelium → plasmogamy ( 2 mycelia fuse) → heterokaryotic stage → karyogamy stage ( fusion of 2 haploid nuclei forming a diploid zygote) → meiosis → spore germination
Asexual reproduction:
Mycelium forms spore-producing structures → molds produce haploid spores by mitosis forming visible mycelia
Fungal infection mechanism
Inhalation of spores or conidia → entry into alveoli → eliciting first line defence → depletion of phagocytic cells leads to disease progression as pulmonary nodules and pneumonia → macrophages phagocyte the fungal cells or encapsulate and from granuloma → fungal cells parasitize the macrophages that lead to vomocytosis of intact fungi and circulation into bloodstream
1. Entry & Initial Defense
Route of Infection: Inhalation of fungal spores/conidia (e.g., Aspergillus, Histoplasma).
First-Line Defense in Alveoli:
PAMP Recognition: Immune cells detect fungal components via PRRs:
PAMPs: β-1,3-glucan, chitin (fungal cell wall).
PRRs: Dectin-1 (for β-glucan), TLRs (e.g., TLR2/TLR4), complement receptors.
Phagocytic Response:
Macrophages/Neutrophils engulf spores → respiratory burst (ROS/RNS) and antimicrobial peptides (AMPs).
Dendritic Cells (DCs) prime adaptive immunity (Th1/Th17 responses).
2. Immune Evasion & Disease Progression
Phagocyte Depletion: Fungal evasion tactics (e.g., melanin in Aspergillus shields PAMPs) exhaust defenses → pulmonary nodules/pneumonia.
Granuloma Formation: Macrophages may encapsulate fungi (e.g., Histoplasma) but fail to kill them.
Vomocytosis: Some fungi (Cryptococcus) survive phagocytosis, escape macrophages intact (non-lytic expulsion), and disseminate via bloodstream → systemic infection.
What are the main types of antifungals and their mechanisms of action?
Azoles (inhibit ergosterol synthesis)
Polyenes (bind ergosterol, forming pores in fungal membranes),
Echinocandins (inhibit beta-glucan synthesis, weakening fungal cell walls).
What drugs affect biosynthesis of ergosterol
Azoles ( Fluconazole, Clotrimazole etc)
Polyenes (nystatin, amphotericin B)
Allylamines (naftifine, terbinafine)
Which drugs affect beta-glucan synthesis?
Echinocandins e.g. Caspofungin, Micafungin
What drugs inhibit DNA replication and mRNA transcription?
Pyrimidine analogues such as pyrimethanil
What are common mechanisms for antifungal and antimicrobial resistance and what are the differences?
Both can form biofilms
Both have efflux pumps, which can secrete the drug out
Both can mutate the target receptor, so drug no longer potent
How do antifungal peptides work
Peptides attach to cell membrane and cause forced entry, where they cause oxidative damage such as by forming hydrogen peroxide, which damage the cell
What are three emerging therapeutics discussed for combating infections?
Mosaic vaccines, CRISPR/Cas9, and mRNA vaccines.
What is the mechanism of action of mosaic vaccines?
Mosaic vaccines use protein nanocages like ferritin, engineered to display multiple antigens on their surface, eliciting a broad immune response against various pathogen strains."
What is the mechanism of action of mRNA vaccines, and how is their mRNA capped?
mRNA vaccines deliver mRNA encoding pathogen antigens to cells, which translate it into proteins to trigger an immune response; mRNA is capped post-transcriptionally using the Vaccinia Capping System or co-transcriptionally with Anti-Reverse Cap Analog (ARCA) or CleanCap Reagent AG to enhance stability and translation efficiency."
What is the purpose of specific structure on mRNA vaccine?
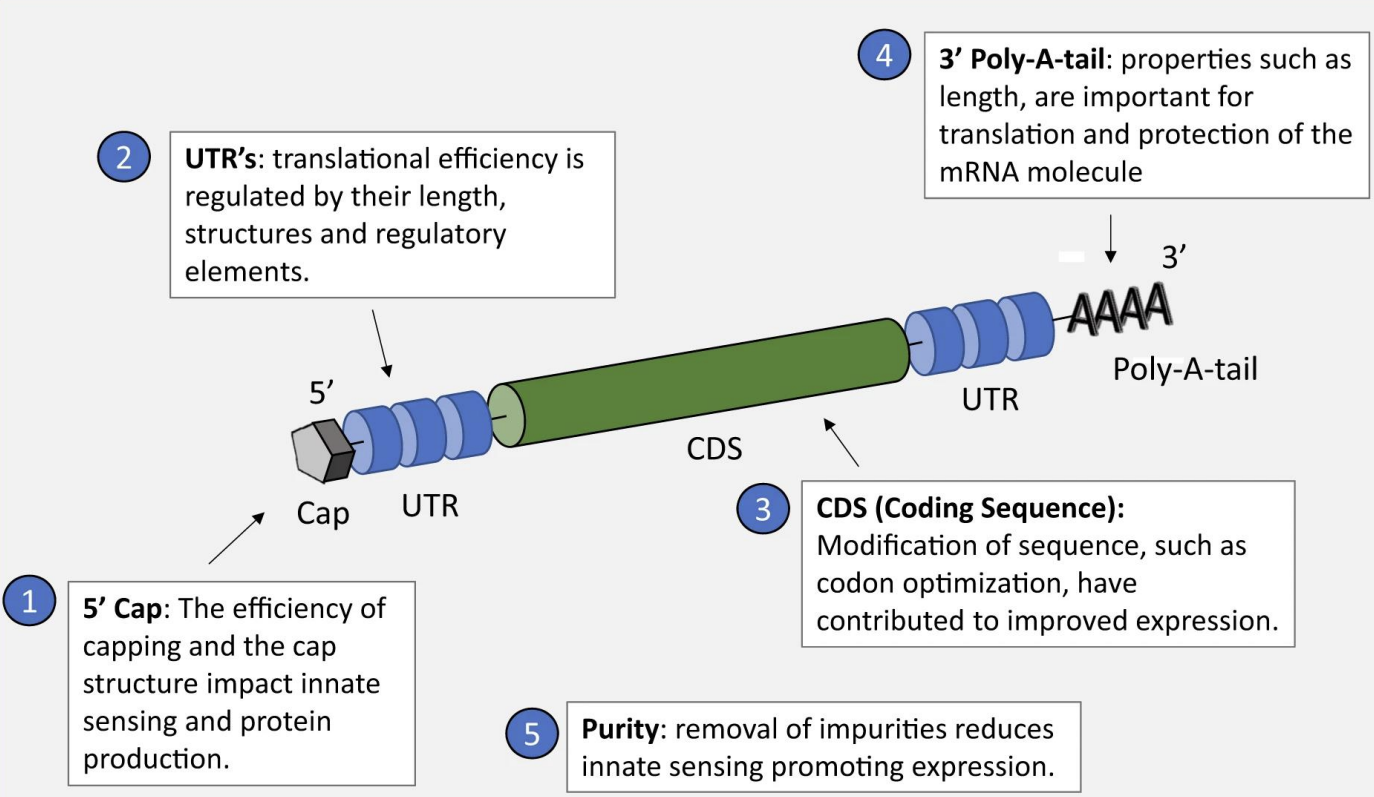
5’ Cap: Prevents Immune Detection: Mimics human mRNA, avoiding recognition as "non-self" by innate immune sensors (e.g., RIG-I). / Ribosome Binding: Essential for translation initiation (eukaryotic ribosomes recognize the cap).
Structure: 7-methylguanosine (m7G) linked via 5’-5’ triphosphate.
Impact:
Inefficient capping → reduced protein production + unwanted immune activation.
—
5’ Untranslated Region (UTR): Regulates Translation Efficiency: Length, secondary structure, and regulatory elements (e.g., Kozak sequence) control ribosome binding. / Optimization Example: Short, unstructured 5’ UTRs enhance translation (used in Pfizer/BioNTech’s COVID-19 vaccine).
—
Coding Sequence (CDS): Codon Optimization: Replace rare codons with host-preferred ones (e.g., human codon bias) to boost protein yield. / Modified Nucleotides: Pseudouridine (Ψ) reduces immune recognition while improving stability.
—
3’ Untranslated Region (UTR): mRNA Stability: Binds proteins that protect against degradation. Translation Regulation: AU-rich elements (AREs) can accelerate decay; optimized UTRs extend half-life.
—-
3’ Poly-A-Tail: Translation Enhancement: Longer tails (~100–150 adenosines) promote ribosome recycling and protein production. / Protection: Shields mRNA from exonucleases, prolonging activity.
Design: Synthetic tails avoid premature shortening (e.g., encoded in DNA template or enzymatically added).
—
Purity: Removal of dsRNA/Impurities: Reduces activation of innate immune sensors (e.g., PKR, TLRs) that inhibit translation.
Methods: HPLC purification, DNase treatment.
5 prime cap= it the body would recognise it as non-self and would kill the vaccine, ribosome recognises it and starts transcription
UTR= translation efficiency is regulated by their length, structure and elements
3 prime poly-A-tail= it's length is important for translation and protection of the mRNA molecule
Explain what are Nanocage protein therapeutics and how they can be used to deliver mosaic vaccines
attach different vaccine subunits to a Ferritin i.e iron storage protein to target different strains
can also put DNA/mRNA inside the storage protein
What is CRISPR-Cas9
CRISPR-Cas9 is part of a bacterial adaptive immune system that protects against pathogens, such as bacteriophages (viruses that infect bacteria).
• When bacteria get infected by bacteriophages, they cut part of the DNA and integrate it in their genome (not lytic or lysogenic cycles) and store it as information ( like memory cells in humans) for next time when they face the pathogen
• When they get infected with the pathogen again, the stored DNA sequences (now transcribed into RNA) are used to produce CRISPR RNAs (crRNAs). These RNAs are
complementary to the pathogen's DNA ( that they initially cut)
• The enzyme Cas9, a nuclease, binds to the crRNA. This RNA guides the Cas9 protein to the matching sequence in the pathogen's DNA.
• Cas9 cuts the pathogen's DNA at the targeted site, preventing its replication and effectively neutralizing the infection.
- this can be used to treat hereditary disorders and treat viral infections e.g. mutating surface receptor CCR5
How does CRISPR/Cas9 function as an emerging therapeutic for infections?
CRISPR/Cas9 edits genes to treat infections by disrupting viral genomes, modifying receptors to prevent viral entry, or targeting pro-viral host factors, as seen in applications against HIV-1."