m5 tuts p1
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
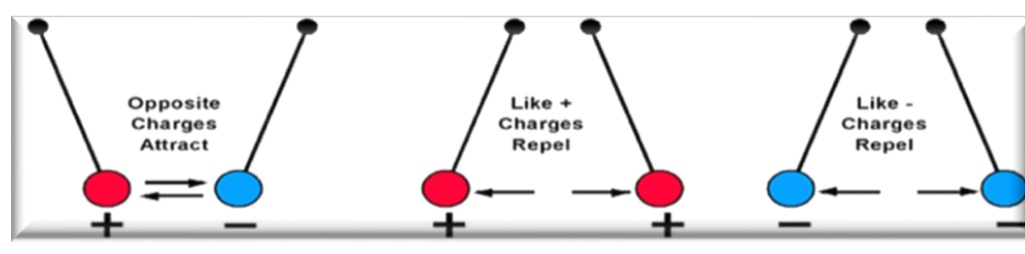
Attractive and Repulsive Forces
Attraction - opposite charge
Repulsion - similar charge (+,+) (-,-)
Cohesive forces
Attraction between identical (similar) molecules
Adhesive forces
Attraction between different molecules
Intramolecular Forces of Attraction
Within a molecule; Na+, Cl- = Na-Cl
Covalent Bond
Coordinate covalent bond
Ionic bond
Covalent Bond
Mutual sharing of electron
Nonmetal + Nonmetal
Nonpolar Covalent Bond
Bonding electrons shared equally (symmetrical distribution of electron) creating no charges on atoms
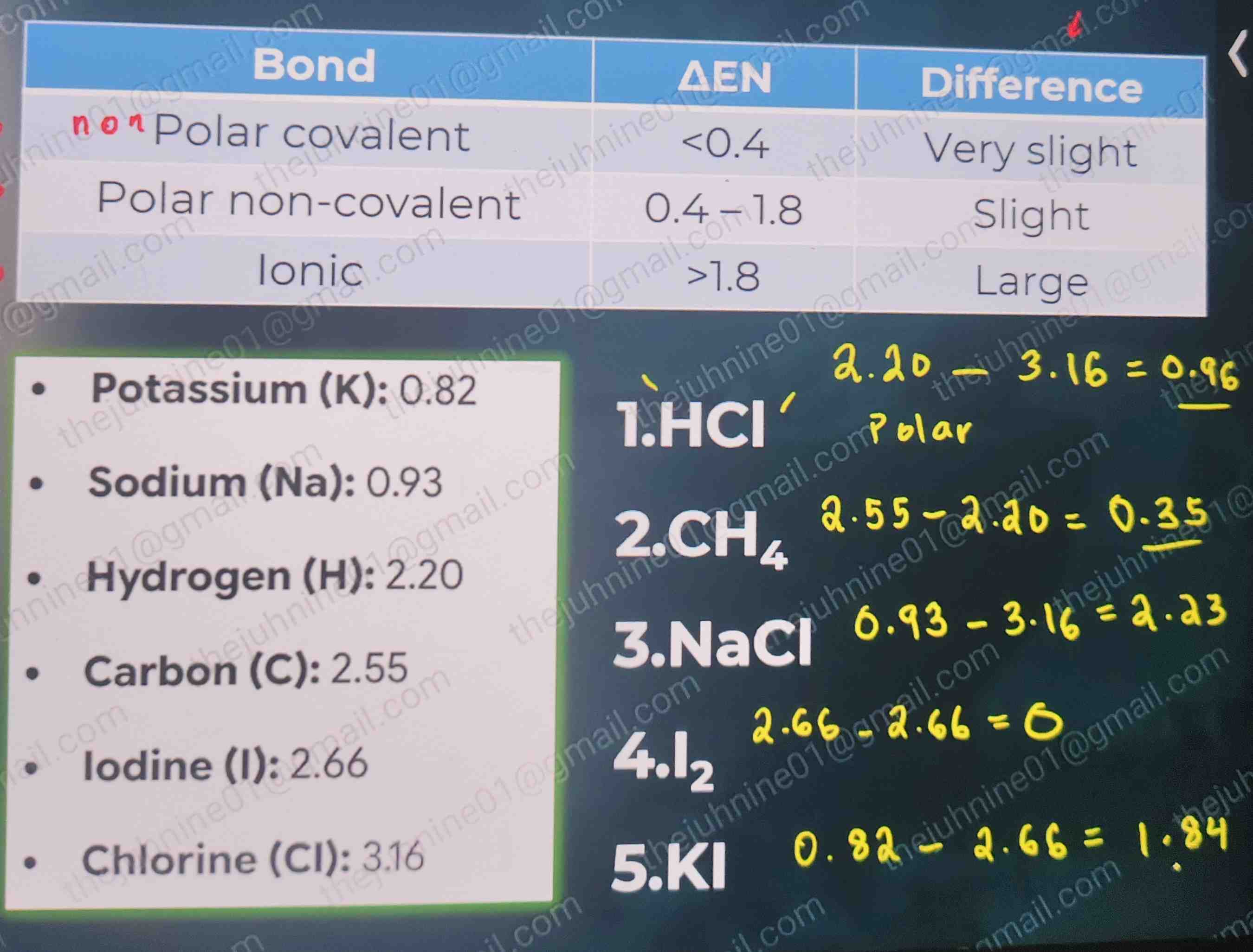
NO or very slight EN difference (<0.4)
C-C (2.55 - 2.55 = 0)
Diatomic molecules (gen-u-ine)
Oxygen, Hydrogen, Nitrogen
Chlorine, Iodine, Fluorine, Bromine (-ine except astatine)
Inert gas (right most part of periodic table)
Polar Covalent Bond
Bonding electrons shared unequally creating partial charges of atoms (asymmetrical)
Slight EN difference (0.4–1.8)
e.g., HCl, H2O
Coordinate Covalent Bond
Non-mutual sharing of electron
Ionic Bond
Metal + Nonmetal
aka: Electrovalent bond
Full charge (cation + anion)
Transfer of electron
Large EN difference (>1.8)
True
HCl - polar covalent bond
CH4 - nonpolar covalent bond
NaCl - ionic bond
I2 - nonpolar covalent bond
KI - ionic bond
True
Covalent - NM + NM
Ionic - M + NM
Metallic - M + M (alloy)
Intermolecular Forces of Attraction
Between molecules (mixture)
Responsible for most of the physical and chemical properties of matter
The stronger the IMF = higher boiling point
Dipole
Polar (partial charge)
Permanent dipole moment (or separation of charge) due to uneven charge distribution
Induced Dipole
Nonpolar (0 charge alone; can have induced polarity - temporary charge, temporary polar)
Temporary dipole moment due to distortion of electron cloud induced by a polar molecule
Ion
Salt
Permanent charge
Van der Waals Forces
NON-IONIC interactions between molecules
WEAKEST of all intermolecular forces of attraction
Van der Waals Forces
Dipole–Dipole
Dipole–Induced Dipole
Induced Dipole–Induced Dipole (London Dispersion Forces)
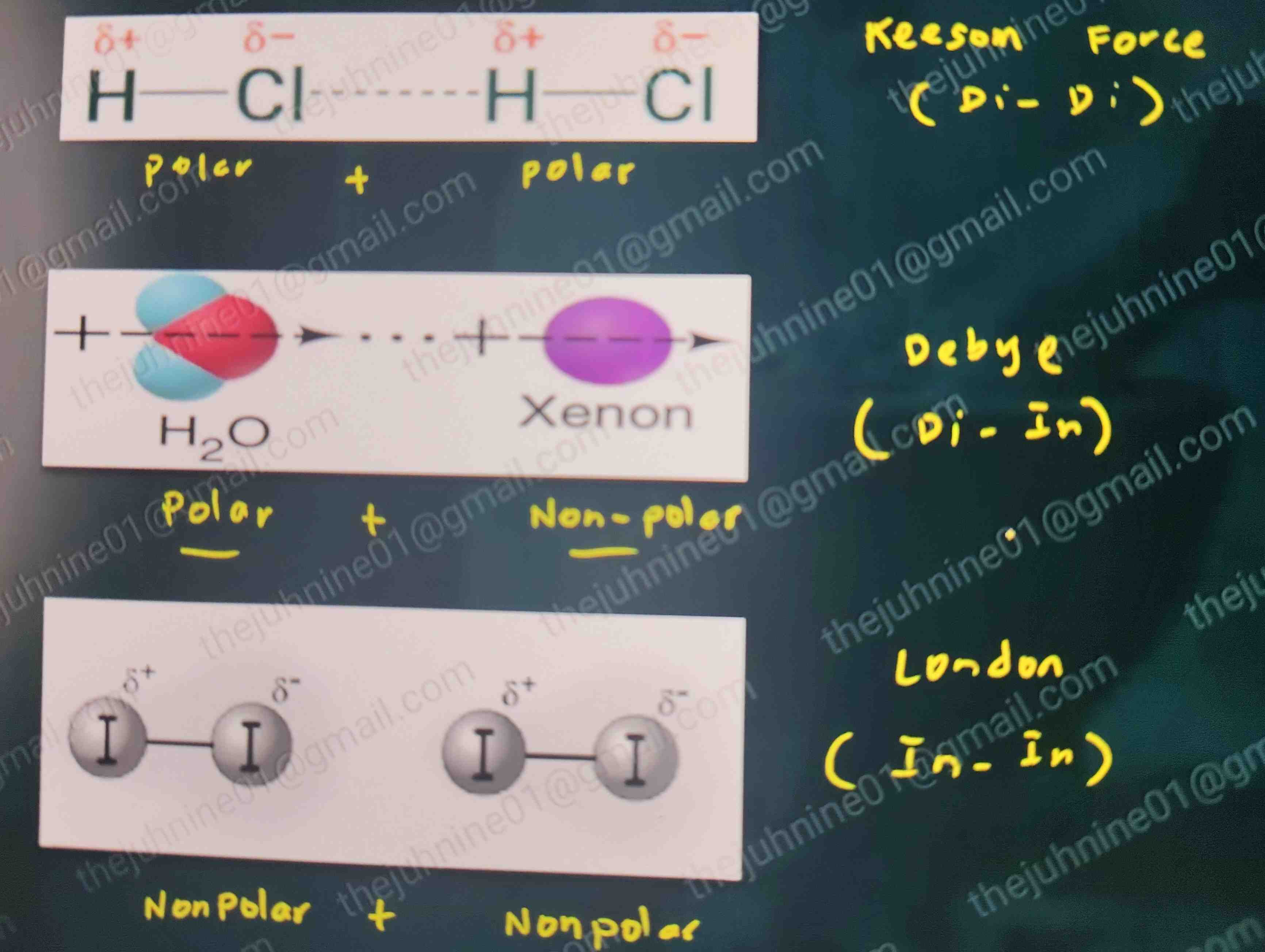
Dipole–Dipole
aka: Keesom Forces
MOA: Alignment or orientation
FOA between: Polar + Polar
Ex: HCl + HCl
Both have a permanent dipole. The slightly positive H of one is attracted to the slightly negative Cl of the other.
Bond strength: 1–7 Kcal/mole
Dipole–Induced Dipole
aka: Debye Forces
MOA: Induction
FOA between: Polar and Nonpolar
Example: H2O (polar) + O₂ (induced dipole)
The polar water molecule, with its slightly negative oxygen and slightly positive hydrogen ends, causes a temporary shift in the electron cloud of the O₂ molecule, inducing a temporary dipole in the O₂.
Bond strength: 1-3 kcal/mol
Induced Dipole–Induced Dipole
aka: London
MOA: Dispersion
FOA between: Nonpolar + Nonpolar
WEAKEST VAN DER WAALS
Bond strength: 0.5-1 kcal/mol
Example: Argon + Argon
The electrons in an argon atom can momentarily shift, creating a temporary dipole. This dipole then induces a dipole in a nearby argon atom, causing a weak, temporary attraction between them.
True
Ion–Dipole
FOA between: Salt and Polar molecule
H2O + NaCl
Na⁺ (positive ion) is attracted to the oxygen side of water (which is negative).
Cl⁻ (negative ion) is attracted to the hydrogen side of water (which is positive).
Ion–Induced Dipole
FOA between: Salt + Nonpolar molecule
I₂ + KI → KI₃
KI dissociates into ions in solution: K⁺ (cation), I⁻ (anion)
I₂ is a nonpolar molecule, meaning its electrons are equally shared.
When I⁻ (iodide ion) approaches I₂, it pushes the electrons in I₂ slightly, inducing a dipole.
This Ion (I⁻) + Induced Dipole (in I₂) creates a weak attraction → forms I₃⁻ (triiodide ion).
KI⁻ (ion) + I₂ (induced dipole) → KI₃⁻ (water soluble)
Hydrogen Bond
aka: Hydrogen Bridge
FOA between: Interaction between a molecule containing a hydrogen atom and a strongly electronegative atom (F, O, N)
H₂O — H₂O (unique: intra and inter)
One water molecule has H–O–H
The oxygen is more electronegative than hydrogen, so:
O gets a partial negative charge (δ⁻)
H gets a partial positive charge (δ⁺)
The δ⁺ hydrogen of one water molecule is attracted to the δ⁻ oxygen of another water molecule
Intramolecular Forces
Intramolecular Forces
A. Polar Covalent
B. Nonpolar covalent
C. Ionic
D. Metallic bond
Transfer of electron → C. Ionic
Equal sharing of electron → B. Nonpolar covalent
Unequal sharing of electron → A. Polar covalent
Metal + Nonmetal → C. Ionic
Alloy formation → D. Metallic bond
Ionic
Transfer of electron
Nonpolar covalent
Equal sharing of electron
Polar covalent
Unequal sharing of electron
Ionic
Metal + Nonmetal
Metallic bond
Alloy formation
Intermolecular Forces of Attraction
Intermolecular Forces of Attraction
A. Van der Waals
B. Ion-dipole
C. Ion-induced dipole
D. Hydrogen bonds
E. Repulsive and attractive forces
F. Electrovalent forces
Keesom, Debye and London forces → A. Van der Waals
Solubility of ionic crystals in water → B. Ion-dipole
Formation of iodide complex, which accounts for the solubility of iodine in KI solution → C. Ion-induced dipole
Accounts for the unusual properties of water (high dielectric constant, abnormally low vapor pressure and high BP) → D. Hydrogen bonds
Forces necessary to cohere and forces necessary to prevent molecular interpenetration → E. Repulsive and attractive forces
Weak electrostatic force that brings about condensation of nonpolar gas molecules → A. Van der Waals (London dispersion forces)
Van der Waals
Keesom, Debye and London forces
Ion-dipole
Solubility of ionic crystals in water
Ion-induced dipole
Formation of iodide complex, which accounts for the solubility of iodine in KI solution
Hydrogen bonds
Accounts for the unusual properties of water (high dielectric constant, abnormally low vapor pressure and high BP)
Repulsive and attractive forces
Forces necessary to cohere and forces necessary to prevent molecular interpenetration
Van der Waals (London dispersion forces)
Weak electrostatic force that brings about condensation of nonpolar gas molecules
Physical Properties of a System
Additive, Constitutive, Colligative
Additive
Depends on sum
Mass or weight
Molecular Weight
Volume
Constitutive
Depends on type and arrangement of molecules
Solubility
Refractive index (Refractometer)
Opitcal activity (Polarimeter)
Viscosity ⭐
Colligative
Depends on the # of solute
Concentration dependent
∆P - Vapor pressure lowering
∆Tb - Boiling point elevation
∆Tf - Freezing point depression
π - Osmotic pressure
Physical Properties of a System
Physical Properties of a System
Properties of drug molecules
A. Electromagnetic radiation
B. Refractive index
C. Optical rotatory dispersion
D. Circular dichroism
E. Permanent dipole moment
F. Optical rotation
Characteristic frequency, wavelength or wavenumber → A. Electromagnetic radiation
Non-ionic phenomenon wherein the molecule has no net charge → E. Permanent dipole moment
Measurement of the angle of rotation → F. Optical rotation
n = sin i / sin r → B. Refractive index
Absorption spectroscopy based on differential absorption of left or right circularly polarized light → D. Circular dichroism
Electromagnetic radiation
Characteristic frequency, wavelength or wavenumber
Increased frequency = shorter wavelength = Greater energy = GAMMA RAY
Decreased frequency = longer wavelength = Lower energy = RADIOWAVES
Permanent dipole moment (Van der waals)
Non-ionic phenomenon wherein the molecule has no net charge
Optical rotation
Measurement of the angle of rotation
Refractive index
n = sin i / sin r
(Incidence, Refraction); Snell's law
Circular dichroism
Absorption spectroscopy based on differential absorption of left or right circularly polarized light
First Law of Thermodynamics
Defines the law of conservation of energy
Energy cannot be created or destroyed
Energy can be transformed; transferred; interconverted
Energy can be interconverted, but the sum of energy must remain constant
Closed system:
∆E = EB – EA
∆E is equal to ZERO for a cyclic process in a closed system
Second Law of Thermodynamics
Defines entropy (S)
Measures the degree of randomness; disorderliness
Gas has greater entropy
Never decreasing; always increasing or constant
The entropy of the system plus that of the surroundings must increase in an irreversible process and remains constant in a reversible process
∆Ssystem + ∆Ssurr ≥ 0
Third Law of Thermodynamics
The entropy of a pure substance is zero when that substance is in a perfectly crystalline state at absolute zero
Gibbs Free Energy
A measure of chemical energy
Represents the combined contribution of enthalpy and entropy
Enthalpy - HEAT CONTENT
Entropy - RANDOMNESS
Formula: ∆G = ∆H – T∆S
Gibbs Free Energy
Interpretation:
∆G < 0 → Spontaneous (negative)
∆G = 0 → Equilibrium
∆G > 0 → Non-spontaneous (positive)
Gas Properties
Indefinite shape
Indefinite volume
High compressibility = spacious
Generally invisible
Exhibit perfect elasticity
Weak intermolecular force of attraction
Molecules move randomly, in constant, rapid motion
Boyle’s Law
Constant: Temperature (BoTe)
P inversely proportional to V
P1V1 = P2V2
Charles’ Law
Constant: Pressure (ChaPre)
Temperature is directly proportional to Volume
Note: Temperature must be expressed in Kelvin (K = °C + 273)
V1/V2 = T1/T2
Ex. Hot air balloon
Gay-Lussac’s Law
Temperature is directly proportional to Pressure
Constant: Volume (Vice Ganda - Gay)
Ex. Aerosol
Combined Gas Law
Combination of Boyle’s law, Charles’ law, and Gay Lussac’s law
Shows the relationship between pressure, volume, and temperature of a gas
Formula: P1V1/T1 = P2V2/T2
Ideal Gas Law
Formula: PV = nRT
n = w ÷ MW
R = ideal gas constant
0.0821 L·atm/mol·K
8.314 J/mol·K
1.987 cal/mol·K (round off to 2)
Remember: J8 2cal
Standard PVT
P = 1 atm; 760 torr/mmHg
V = 22.4 L
T = 273 K; 0°C
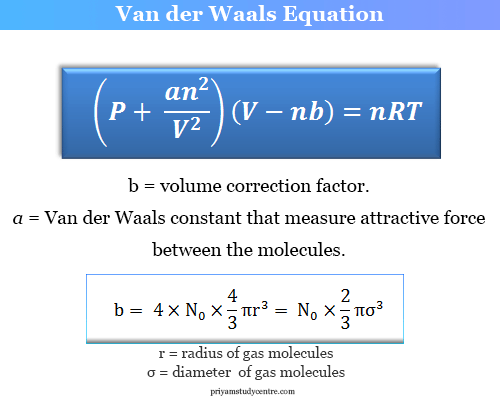
Real Gas Law
Aka Van der Waals equation
Modification of ideal gas law
Henry’s Law
Constant: Temperature
AMOUNT OF DISSOLVED GAS is DIRECTLY proportional to the PARTIAL PRESSURE of that gas in equilibrium with that liquid
Formula: P = KH × C
Soda is bottled under high pressure to keep CO₂ dissolved. When you open it, pressure drops → gas escapes → fizz!
Avogadro’s Law
VOLUME is DIRECTLY proportional to AMOUNT OF GAS (mole)
Constant: Temperature and Pressure (ATP)
Graham’s Law
Effusion or diffusion rate is inversely proportional to the square root of molar mass (or density).
High molar mass → slower effusion
Low molar mass → faster effusion
Helium - lighter, Air - heavier
Dalton’s Law of Partial Pressure
Ptotal = sum of partial pressures
Formula: P1 + P2 + P3 + …..
Where:
P1,P2,P3,… are the partial pressures of the gases.
The pressure a single gas would exert if it were alone in the container.
B. Van der Waals forces
Identify the weak intermolecular forces, examples of which are the Keesom-Debye, and London forces.
A. Ion-dipole, ion-induced dipole forces
B. Van der Waals forces
C. Hydrogen bonds
D. Repulsive and attractive forces
C. Ion-dipole, ion-induced dipole forces
Identify the forces that account in part for the solubility of ionic crystalline substances in water and are presumed to account for the solubility of iodine in a solution of KI.
A. Hydrogen bonds
B. Repulsive and attractive forces
C. Ion-dipole, ion-induced dipole forces
D. Electrovalent forces
B. Van der Waals forces (London)
Identify the weak electrostatic forces that bring about condensation of nonpolar gas molecules to form liquids and solids when molecules are brought quite close to one another.
A. Hydrogen bonds
B. Van der Waals forces
C. Ion-dipole, ion-induced dipole forces
D. Repulsive and attractive forces
A. Gibbs Free Energy
Identify the Law/Principle which states that when the reaction is reversible, ∆G = 0.
A. Gibbs Free Energy
B. Ideal Gas Law
C. Henry’s Law
D. Faraday’s Law
D. ∆E = EB + EA (minus)
Identify the statement/mathematical expression, which does NOT express the First Law of Thermodynamics.
A. States that energy is conserved
B. Forms of energy can be interconverted, but the sum of energies remains constant
C. ∆E is equal to zero for a cyclic process in a closed system
D. ∆E = EB + EA
A. 3rd Law of Thermodynamics
Select the concept that explains the thermodynamics state of a perfect crystal at absolute zero.
A. 3rd Law of Thermodynamics
B. 2nd Law of Thermodynamics
C. 1st Law of Thermodynamics
D. 1st, 2nd, 3rd Laws of Thermodynamics
A. 1.24
If 0.55 g of a gas dissolves in 1.0 L of water at 2 atm of pressure, how much will dissolve at 4.5 atm? (Henry - direct)
A. 1.24
B. 0.55
C. 0.01
D. NOTA
C. 819 mL
A 600 mL sample of nitrogen is warmed from 200 K to 273 K. Find its new volume if the pressure remains constant. (Charles - direct)
A. 150 mL
B. 500 mL
C. 819 mL
D. NOTA
C. Pressure
According to Boyle’s Law, the volume of a gas has an inverse relationship with ____.
A. Moles
B. Temperature
C. Pressure
D. Weight