General Chemistry Midterm Review: Reactions, Calculations, and Lab Procedures
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
77 Terms
Major component of Nature gas
Methane
Ternary acid H2SO4
sulfuric acid
Formula of sodium hydrogen carbonate
NaHCO3
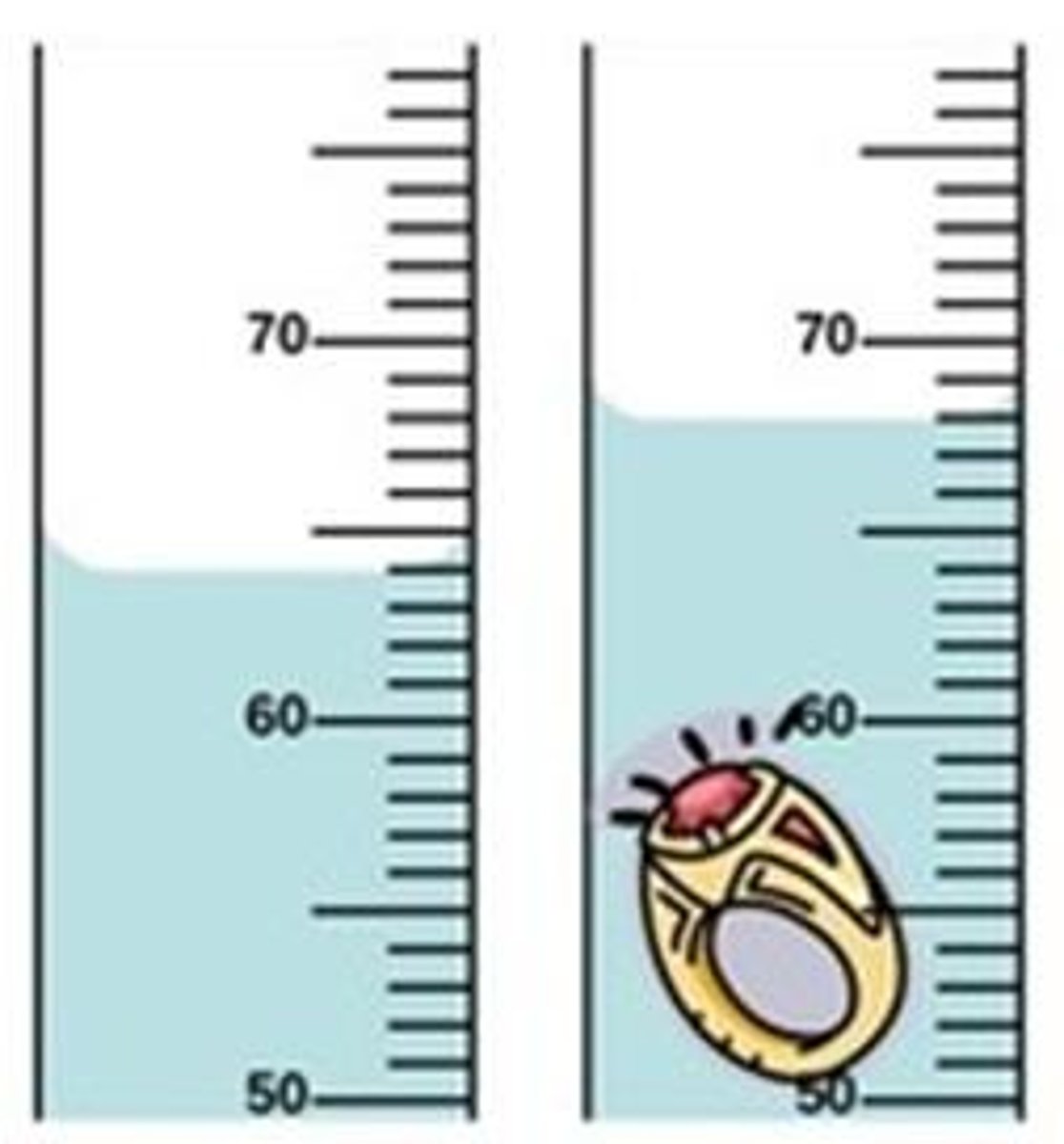
Volume read from graduated cylinder
76.0 mL
Density of gold
19.3 g/cm3
Reason for using crucible tongs
To avoid contaminating the crucible and lid with the contaminants on the skin of the fingers and avoid burning the fingers.
Best way to cool crucible and lid
In a desiccator to minimize the probability of water being adsorbed onto the crucible and lid.
Preparation of Mg ribbon before reaction
To be polished
Molar mass of FeSO4
151.908 g/mol
Empirical formula of table salt
NaCl
Empirical formula of silver sulfide
Ag2S
Type of reaction A + B = AxBy
Combination Reaction
Limiting reactant
The reactant that determines how much product can be made
Correct statement about chemical reactions
A chemical reaction stops when the limiting reactant is used up
Moles of Mg needed to react with 12 mol of HCl
6 moles
Limiting reagent in gas mixture of H2 and O2
To be determined based on the reaction equation
Limiting Reagent
In the reaction 2H2(g) + O2(g) → 2H2O(g), the limiting reagent is O2.
Yield Percentage
In the experiment with 2 moles of H2 and 30 g of H2O generated, the yield percentage is 83.3%.
Purpose of Gravity Filtration
The purpose of gravity filtration is to separate precipitate from liquid solution.
Moles of Li3N Yielded
For the reaction 6 Li(s) + N2(g) → 2 Li3N(s), if 3 mol of Li(s) mixed with 1 mol of N2(g), 1 mol of Li3N(s) is yielded.
Color of Copper (II) Ion
The color of the copper (II) ion in solution is (B) sky blue.
Balanced Equation for Displacement
The balanced equation for the displacement of copper(II) ion from solution with magnesium metal is Cu2+(aq) + Mg(s) → Cu(s) + Mg2+(aq).
Reaction Type
The reaction 2NaOH (aq) + Cu(NO3)2(aq) → Cu(OH)2 (s) + 2 NaNO3(aq) is a Double displacement reaction.
Purpose of Centrifuge
The purpose of a centrifuge is to separate the two substances.
Percent Recovery of Copper
If a 0.0152 g sample of copper metal is recycled and 0.0076 g is recovered, the percent recovery of the copper metal is 50%.
Ions in K2CO3 and FeCl3
The ions present in a mixture of K2CO3 and FeCl3 before any reaction occurs are K+, CO32-, Fe3+, Cl-.
Law of Conservation of Mass
According to the Law of Conservation of Mass, when you burn a candle, the rest of the original candle's mass was changed to gases.
Type of Reaction
The reaction 4Si + S8 --> 2Si2S4 is a Combination reaction.
Spectator Ions
Spectator ions appear in the total ionic equation for a reaction, but not in the net ionic equation. (True)
Oxidizing Agent
If oxygen is an oxidizing agent in a reaction, the oxidation number of oxygen must be decreased.
Oxidizing and Reducing Agents
In the reaction Zn (s) + Fe2+ → Zn2+ + Fe (s), the reducing agent is Zn.
Redox Reaction
The reaction Mg (s) + CuCl2 (aq) → MgCl2 (aq) + Cu (s) is a Redox Reaction.
Oxidation Process
The chemical change Mg(s) → Mg2+ + 2e- is an Oxidation process.
Bunsen Burner
How to operate: The order to open valves and ignite.
Air-control valve
Color changes with/without sufficient air.
Cool flame
A flame produced with insufficient air.
Hot flame
A flame produced with sufficient air.
Density
D = M/V
Unknown solid measurement
How to measure volume of unknown solid and calculate density.
Density calculation effect
If the solid does not immerse into water completely, the recorded volume will be lower than the real volume.
Density calculation
Based on equation; D = M / V, the measured V ↓, therefore the calculated D ↑.
Unknown liquid density
How to calculate density and errors due to liquid evaporation.
Graduated cylinder
How to read volume using graduated cylinder.
Significant Figures
Follow Sig Fig rules to do calculation.
Combination Reaction
Reaction of Magnesium and Oxygen.
Chemical equation for Magnesium and Oxygen
2Mg + O2 → 2MgO
Molar Mass of Mg
24.32 g/mol
Molar Mass of O
16 g/mol
Crucible usage
How to use crucible when heating Mg.
Dry agent
Substance that causes color change.
Concept of Mole
Understand concept of Mole.
Empirical Formula calculation
Calculation from data as in Report sheet.
Mass percent calculation
Calculation from data as in Report sheet.
Balancing equations
How to balance the equation.
Excess Reactant
The reactant that is not completely consumed in a reaction.
Yield percentage calculation
Yield percentage = actual yield / theoretical yield * 100%
Theoretical yield
The maximum amount of product that can be formed from given amounts of reactants.
Example yield calculation
If 3 mol of Li(s) mixed with 1 mol of N2(g), how many mol of Li3N(s) yielded?
Balanced Equation
6 Li(s) + N2(g) → 2 Li3N(s)
Molar ratio
Li : N2 : Li3N = 6 : 1 : 2
Limiting Reactant (LR)
The reactant that is completely consumed in a reaction, determining the amount of product formed.
Excess Reactant (ER)
The reactant that remains after the limiting reactant is used up in a reaction.
Percent of Na2SO4 (LR)
Mass percent of Na2SO4 (LR) = 0.284g / 0.810 g X 100% = 35.1%
Percent of BaCl2•2H2O (ER)
Mass percent of BaCl2•2H2O (ER) = 0.526g / 0.810 g X 100% = 64.9%
Mole Calculation
The mole of BaSO4 is: Mass/Molar Mass = 0.00199 mol
Recovery Percent
A calculation to determine the efficiency of a reaction, often expressed as a percentage.
Reduction Process
The process that one substance gains electrons.
Reducing Agent
A substance that donates electrons in a redox reaction, causing another substance to be reduced.

Double Displacement Reaction
A type of reaction where two compounds exchange ions to form two new compounds.
Decomposition Reaction
A reaction where a single compound breaks down into two or more simpler products.
Molecular Equation
An equation that shows the complete chemical formulas of reactants and products.
Complete Ionic Equation
An equation that shows all of the ions present in a solution.
Net Ionic Equation
An equation that shows only the ions that participate in the reaction, excluding spectator ions.
Chemical Equations for Reactions
Cu → Cu(NO3)2 → Cu(OH)2 → CuO → CuSO4 → Cu
Molar Mass of BaCl2•2H2O
244.27 g/mol
Molar Mass of Na2SO4
142.04 g/mol
Molar Mass of BaSO4
233.39 g/mol