Chemistry 2: Thermodynamics and Gasses
1/19
Earn XP
Description and Tags
Test 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Density of a Gas
(Molar Mass)*(P/RT)
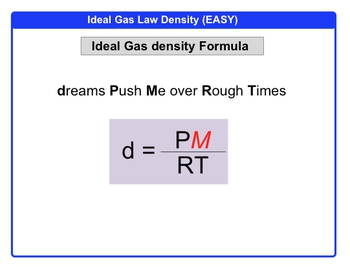
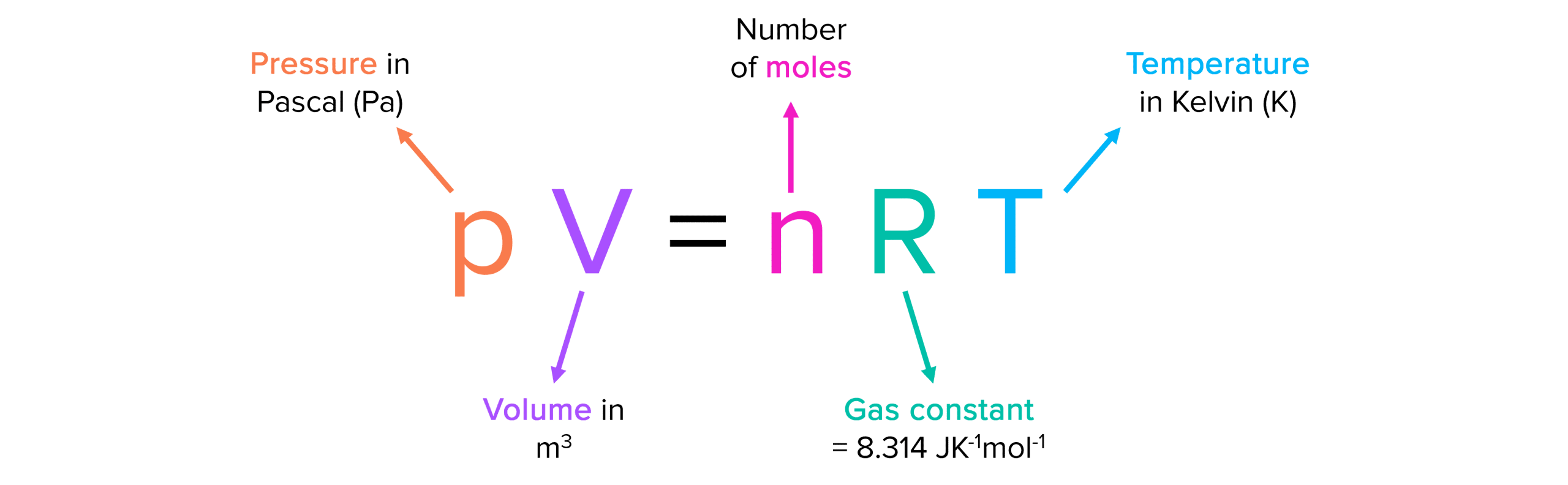
Ideal Gas Equation
PV=nRT
Effusion Rate Formula
r1/r2=sqrt(a2/a1)
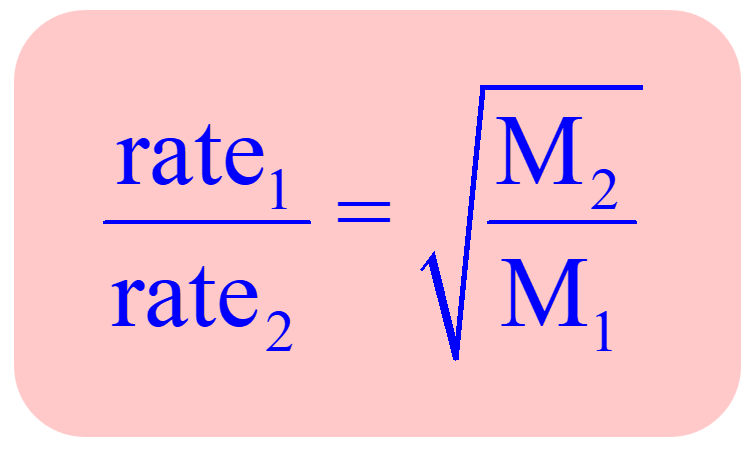
Mole Fraction
Moles of part/Moles total
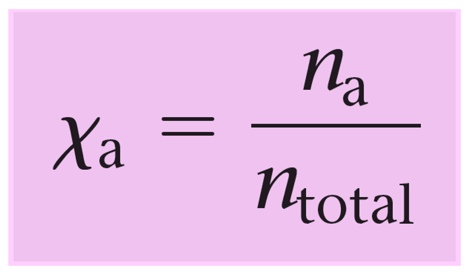
Partial Pressure
(mole fraction)*(Total pressure)
Kinetic Energy
1/2(mv²)
Specific Heat Capacity
q=mC𝚫T
Exothermic
releases
Endothermic
absorbs
Energy Change
𝚫E = EF - EI
Law of Conservation of Energy
total energy of the universe is constant(always 0) and it can’t be destroyed
Heat Vs Temperature
Heat: Extensive Temperature: intensive
Enthalpy
𝚫H—state function
state function
independent of path
Hess’ Law
if a reaction is carried out in steps, the total enthalpy is equal to that of the steps added
non-ideal gas
low temp/high preassure
At the same temperature gasses
have the same kinetic energy
KE of particles in the system
KE: (3/2)RT
1atm
760 mmhg or torrs
0 C
273.15 K