Compounding Final
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
What is the amount (in gram) of concentrated HCl required to prepare 1000 ml of diluted acid (10% w/v)?
Concentrated HCl - is 37.5% w/w
Diluted HCl - is 10% w/v
267 g
What is the amount (in mL) of concentrated HCl (Sp. Gr. = 1.18) required to prepare 1000 ml of diluted acid (10% w/v)?
Concentrated HCl - is 37.5% w/w
Diluted HCl - is 10% w/v
226 mL
How many grams KMnO4 should be used in preparing 6 oz of a prescription such that two teaspoonfuls placed in 8 oz of water gives 1:5000 concentration of KMnO4?
0.9 g
How many milliliters of a 17% (w/v) KMnO4 should be used in preparing 6 oz of a
prescription such that two teaspoonfuls placed in 8 oz of water gives 1:5000
concentration of KMnO4?
5.29 mL

Rifampin - 1800 mg (or 1.8 g ) needed
Sodium Carboxymethylcellulose - 1.2 g
How many milliliters each of 2% iodine tincture and 7% strong iodine tincture should
be used in preparing 1 gallon of a tincture containing 3.5% of iodine?
2% tincture needed = 2650 mL
7% tincture needed = 1135 mL
How many grams of 2.5% hydrocortisone cream should be mixed with 360 g of
0.25% cream to make a 1% hydrocortisone cream?
180 g
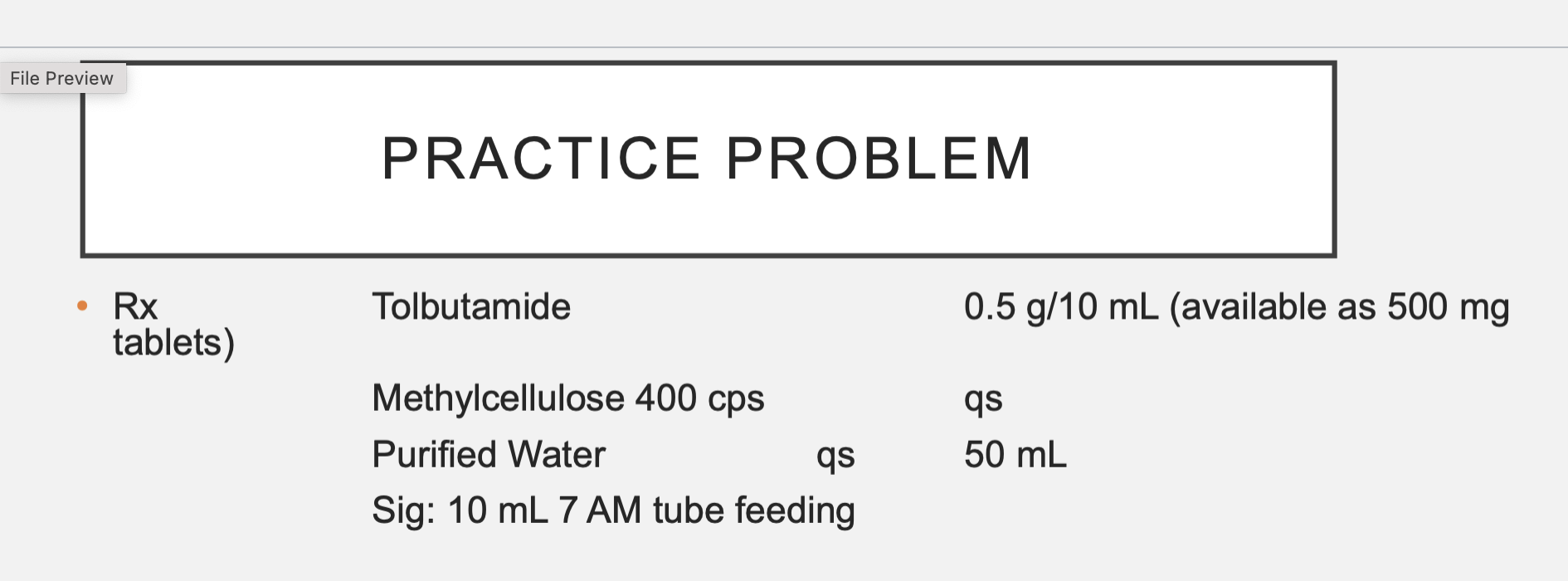
Tolbutamide = 2.5 g or 2500 mg, 5 tabs needed
Methycellulose = 0.5
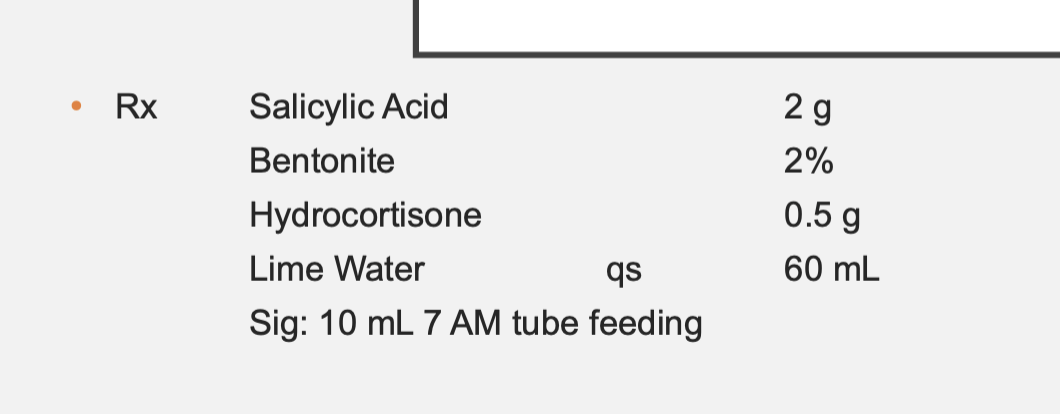
Bentonite comes available as bentonite magma 5%
Bentonite = 1.2 g needed, 24 mL needed to get 1.2 g of bentonite from the stock magma solution

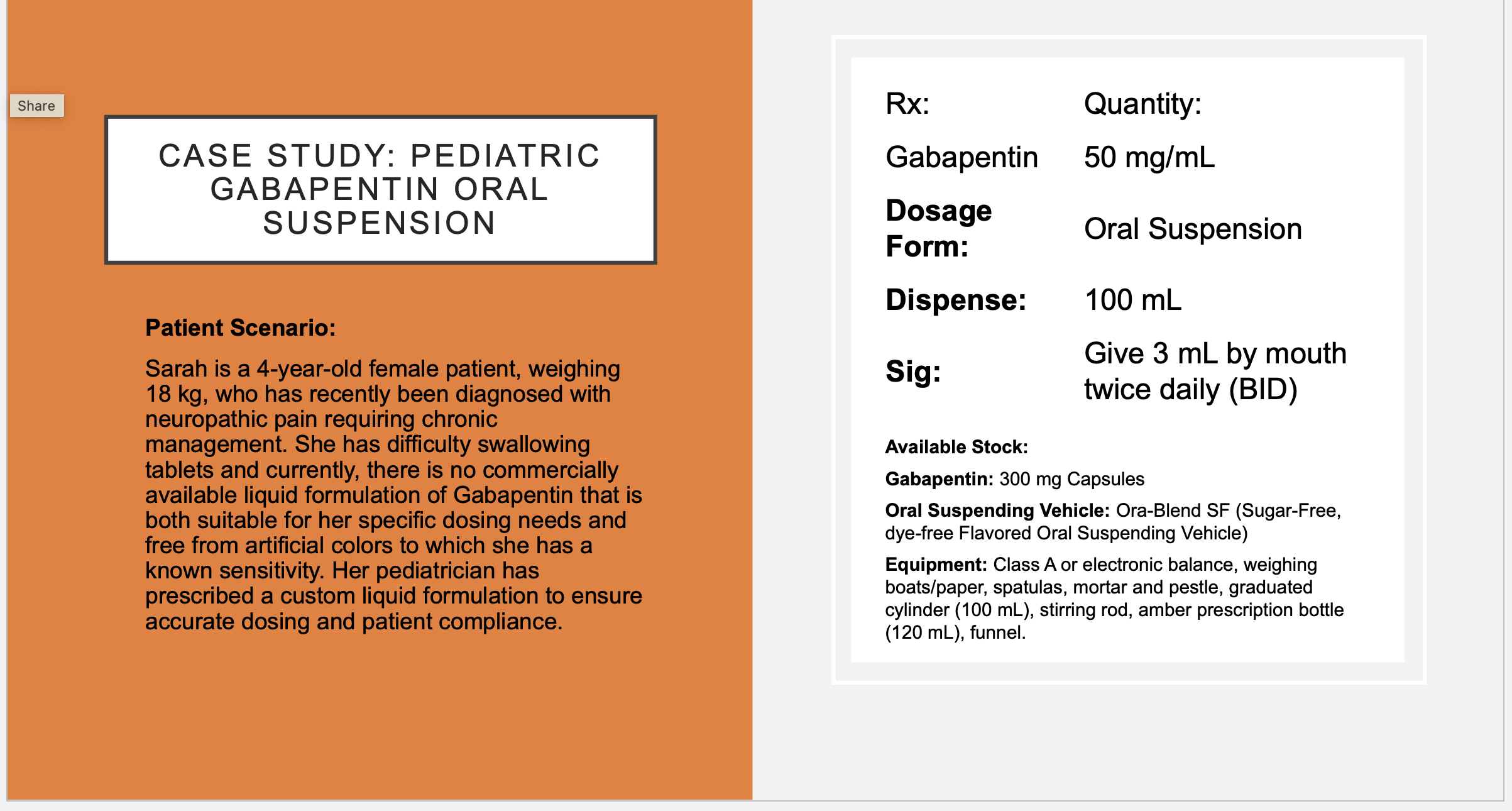
Mg Gabapentin needed = 5000 mg
Caps needed to get 5000 mg = ~17 capsules

aa = of each
Menthol and Thymol = 1.5 g each
Pet Alba and Zinc Oxide Paste = 13.5 g each

Zinc Oxide = 10 g
Mineral Oil = 7.5 g
White Petrolatum = 32.5 g
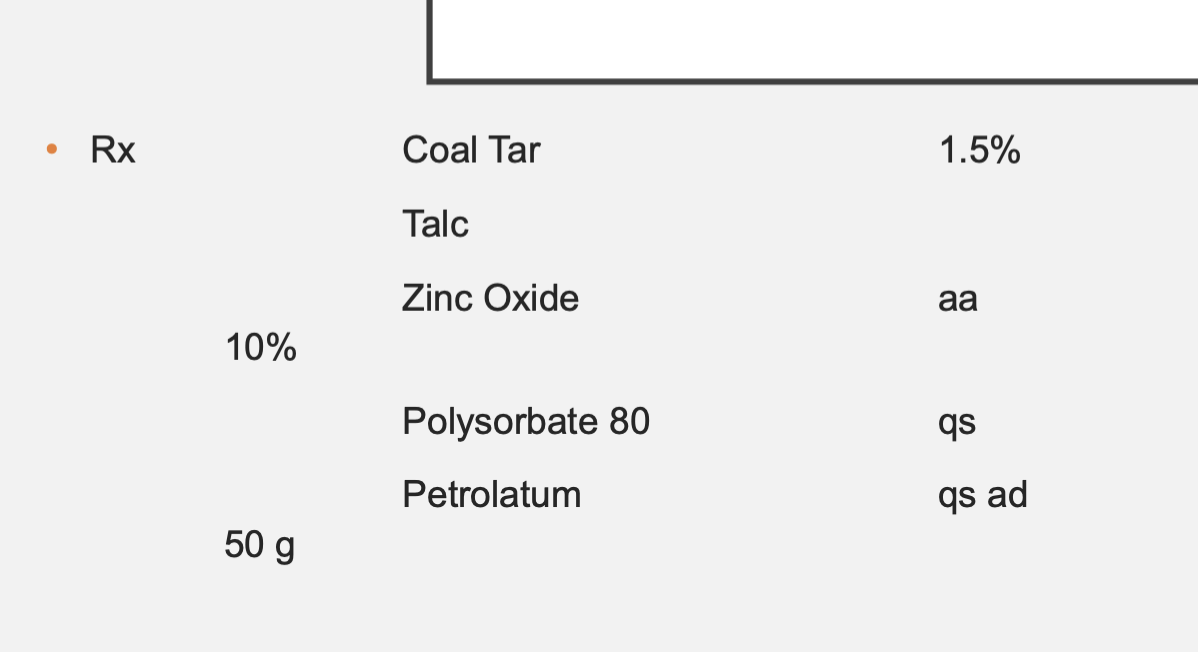
Coal Tar = 0.75 g
Talc and Zinc Oxide = 5 g each
Polysorbate 80 = we can’t know how much to use until we compound the preparation. Determined while compounding
Petrolatum = 44.25 g
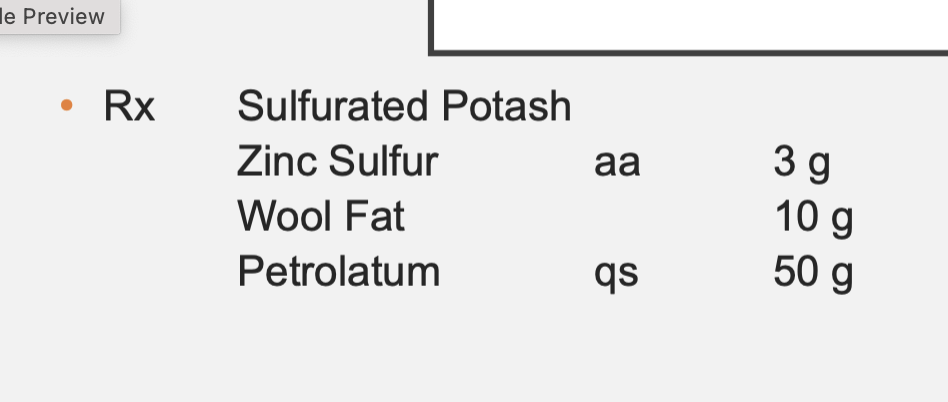
Sulfurated Potash = 3g (aa of Zinc Sulfur below it)

Choice of Levigating agent left to the pharmacist —> must use Mineral oil


29.7 g (double check)

69 g
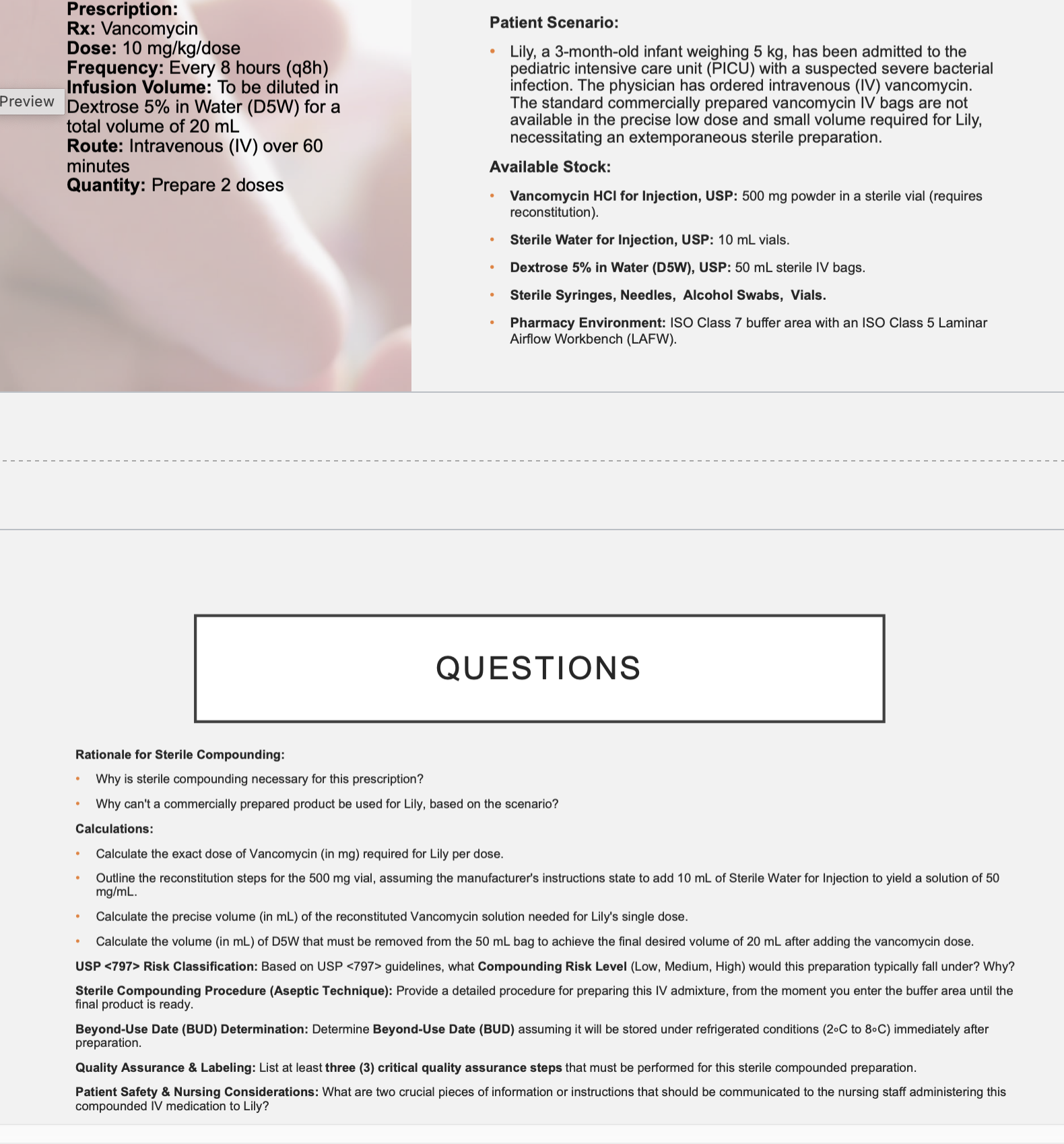
A 25-year-old female major trauma patient survives surgery and is
recovering in the surgical intensive care unit. The medical team wants to
start PN therapy. She is 122 pounds, 5’7’’ with some mild renal
impairment. Calculate her BEE using the Harris-Benedict equation and
her Calorie goal (modifier 1.2)
1646 kcal
Potassium (K+) has a gram-atomic weight of 39.10. The valence of K+ is 1. Calculate its milliequivalent weight.
39.10 mg
Calcium (Ca++) has a gram-atomic weight of 40.08. The valence of Ca++ is 2.
Calculate its milliequivalent weight.
20.04 mg
Calculate the milliequivalent weight of potassium in a potassium salt of an antibiotic,
having a molecular weight of 388.48 g per mole. There is one potassium atom in the
molecule, and the valence of potassium is 1.
388.48 mg
Calculate the milliequivalents of potassium contained in 250 mg of a tablet of a
potassium salt of an antibiotic, having a molecular weight of 388.48 g per mole.
There is one potassium atom in the molecule, and the valence of potassium is 1.
0.64 mEq
How many mL of normal saline (MW=58.5) will provide 8 mEq of sodium?
52 mL
How many milliequivalents of lithium (Li+) are contained in six 300 mg tablets of
lithium carbonate (MW=74)?
48.65 mEq
Calculate the number of milliequivalents of magnesium sulfate, magnesium ions, and sulfate ions present in 1 g of magnesium sulfate (MgSO4 7H2O; MW=246).
For magnesium sulfate:
For magnesium ions:
For sulfate ions:
Valence of the whole salt is 2
For magnesium sulfate: 8.13 mEq
For magnesium ions: 8.13 mEq
For sulfate ions: 8.13 mEq
How many milliequivalents of Na+, Cl-, and NaCl are represented in 100 mg of NaCl (MW=58.5)?
NaCl has a valence of 1
NaCl = 1.7 mEq
Na = 1.7 mEq
Cl = 1.7 mEq
How many milliequivalents of aluminum and carbonate ions are represented in 100
mg of aluminum carbonate, Al2(CO3)3 (MW=234)?
Al = 2.56 mg
CO3 = 2.56 mg
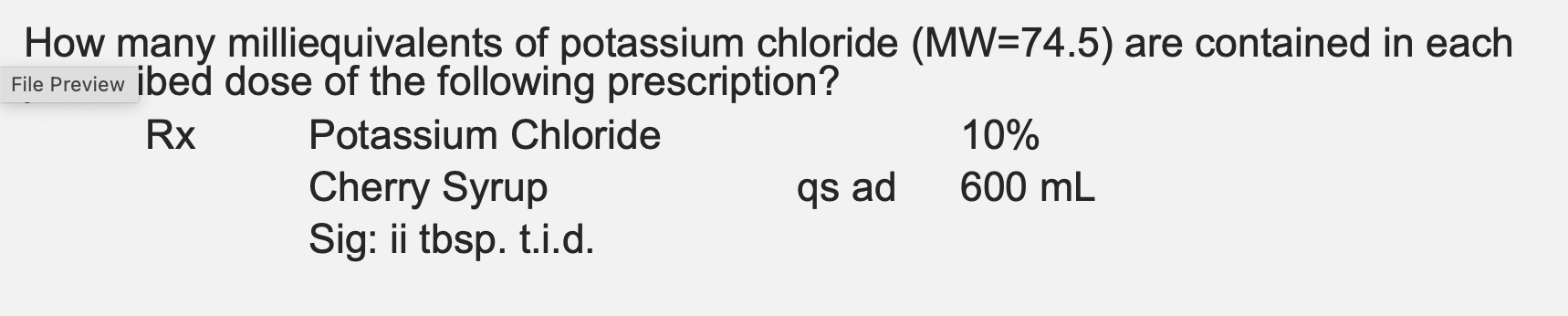
How many milliequivalents of potassium chloride (MW=74.5) are represented in a
solution containing 1.49 g of potassium chloride per liter of solution?
KCl has a valence of 1
20 mEq
How many milliequivalents of potassium chloride (MW=74.5) are represented in a
solution containing 0.155 g of potassium chloride per 100 mL of solution?
2.08 mEq
What is the percent concentration of a solution containing 200 mEq of sodium chloride (MW=58.5) per liter of solution?
1170 mg —> 11.7 g
= 1.17%
What is the concentration, in g/L, of a solution containing 4 mEq of calcium chloride
(CaCl2 2H2O) (MW=147) per mL of solution?
588 mg
How many mEq of calcium chloride (CaCl2 2H2O) (MW=147) are represented in 735 mL of a 5% solution of calcium chloride?
500 mEq
If 20 mL of an electrolyte solution containing 0.25 mEq of Ca++/mL is administered in 500 mL of 0.45% sodium chloride (MW=58.5) solution, how many mEq of sodium
does the patient receive?
38.46 mEq
40.27 mEq per dose
A 2 g vial of an antibiotic yields a concentration of 250 mg/mL when reconstituted with 7.2 mL of Sterile Water for Injection. Calculate the volume of reconstituted solution needed to provide a dose of 300 mg.
A 3 g vial of an antibiotic yields a concentration of 200 mg/mL when reconstituted with 7.2 mL of Sterile Water for Injection. Calculate the volume of reconstituted solution needed to provide a dose of 300 mg.
1.5 mL
A medication order calls for 1000 mL of D5W to be administered over 8-hour period.
Using an IV administration set that delivers 10 drops/mL, how many drops per minute should be delivered to the patient?
20.8 drops → 21 drops
Ten (10) milliliters of 10% calcium glucose injection and 10 mL of multivitamin
infusion are mixed with 500 mL of a 5% dextrose injection. The infusion is to be
administered over 5 hours. If the dropper in the venoclysis set calibrates 15
drops/mL, at what rate, in drops per minute, should the flow be adjusted to
administer the infusion over the desired time interval?
26 drops
The IV dose of ondansetron is three 0.15 mg/kg doses infused over 15
min.
1) What would be the initial dose (in mg) for a patient weighing 134
pounds?
2) If the dose is diluted to 50 mL with D5W, what flow rate in mL/hr,
would be needed to administer the dose over 15 min?
3) What flow rate in drops/min would be needed using an infusion set
that delivers 20 drops/mL?
An intravenous infusion contains 10 mL of a 1:5000 solution of isoproterenol hydrochloride and 500 mL of a 5% dextrose injection. At what flow rate (in mL/min) should the infusion be administered to provide 5 mcg of isoproterenol hydrochloride per minute, and what time
interval (in min) will be necessary for the administration of the entire infusion?
A physician’s medication order calls for 400 mg of clindamycin to be
added to 600 mL of D5W for IV infusion over 90 min. Clindamycin is
available as an injection containing 600 mg/4 mL.
(a) How many milliliters of the clindamycin injection should be used,
(b) how many mg/ml of clindamycin will the infusion contain, and
(c) how many milliliters per min of the infusion should be delivered?
If the volume of the drug solution added to IV bag is < 10%, do not add up
the volume
An intravenous infusion contains 10 mL of a 1:5000 solution of isoproterenol
hydrochloride and 500 mL of a 5% dextrose injection. At what flow rate should the
infusion be administered to provide 5 μg of isoproterenol hydrochloride per minute
and what time interval will be necessary for the administration of the entire infusion?
There are 2000 mcg in the 10 mL soln
1.25 ml per min to administer 5 mcg of isoproterenol per min
If 1.25 ml are given per min, it takes 400 min to administer the entire 500 mL bag. (the 10 mLs added are not accounted for here)

