OCR A-level Biology Paper 1
1/968
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
969 Terms
Practical 1 : Using a Light microscope to examine blood smears
Preparation:
1.) staining samples with methylene blue/ eosin
- staining to add contrast so it is easier to identify different parts of te cell
- for the electron microscope, the specimens are stained with lead
2.) mounting
- dry mount:
thinly sliced
use tweezers to pick up your specimen and put it in the middle of a clean slide
place a coverslip over the top of the specimen
- wet mount:
1.pipette a small drop of water onto the slide
2.use tweezers to place the specimen on top of the water drop
3.stand the coverslip upright on the slide next to the water droplet 4.and carefully tilt and lower the cover slide so it covers the 5.specimen to avoid air bubbles which may obstruct view.
6.stain the specimen by putting a drop of the stain next to the cover slip.
Microscope
1. clip the slide containing the specimen onto the stage
2. select lowest powered objective lens
3. use coarse adjustment knob to bring the stage up to just below objective lens
4. look down the eyepiece and the coarse adjustment knob to move the stage so the image is roughly in focus.
5. adjust the focus with the fine adjustment.
knob until a clear image is obtained
6. swap to greater magnification with higher-powered objective lens
REMEMBER
- MAGNIFICATION = IMAGE SIZE / OBJECT SIZE
- stage micrometer is the bigger 'ruler'
- eye piece graticule is the smaller 'ruler'
Practical 2 : Dissection of a mammalian heart
equipment: pig's heart, dissecting tray, scalpel, spron and lab gloves
external examination:
- try to identify four main vezzels
-arteries are thick and rubbery
- veins are thinner
- identify the right and left atria
internal examination
- cut along the lines
- measure and record the thickness of the ventricle walls note any differences ventricular walls
- cut open the atria and look inside them too.Note whether atria walls are thicker or thinner than the ventricular walls
- find AV valves and semi-lunar valves.
- Draw sketch to show the valves
Practical 6: The effect of substrate/enzyme concentration on enzyme controlled reaction *catalase
For investigating different enzyme and substarte concentrations.
1. set up boiling tubes containing same volume and concentration of substrate (or enzyme)
2. set up boiling tubes containing different concentrations of enzymes (or substrate)
3. add equal volumes of buffer solution
4. set up apparatus to measure the gas given off for colour change
5. time how long it takes for changes to happen
* for temperature investigation use water bath
* for pH investigation pre-measure pH and use different pH for each test (don
*keep pH and temperature constant
Practical 7: Investigating the effect of temperature on amylase activity
1. add equal volumes of iodine drops (amylase breaks down starch) into dropping tiles
equal volumes of starch solution and amylase
2. same volume and concentration of amylase and starch solutions incubated in a water bath at set intervals on temperature for the same amount of time until the desired temperature is reached
3. add amylase enzyme to starch and iodine
4.time how long it take for when the iodine solution in the spotting tile remains orange-brown (no starch is being broken down).
variable that need to be controlled
- pH
- temperature
-enzyme concentration
- substrate concentration
When enzyme from natural source how to control experiment better
-same plant
-same part of plant
-same mass of tissue
-same size
*Practical 8: The effect of temperature on membrane permeability (beetroot experiment)
1. cut equal size pieces of beetroot and rinse them to remove any pigment released during cutting.
2. Place the five pieces in five different test tubes, each with the same volume of water
3. Place each test tube in a water bath set at different temperatures for the same length of time
4 remove the pieces of beetroot from the test tubes, leaving just the coloured liquid
5. take the small volume of each coloured liquid and put it into covet for the colorimeter
*make sure to calibrate the colorimeter with a blank first
6. measure the absorbance rate of each liquid
7. the more absorbance (less light passing through) the more permeable the membrane was
* more transmission = less permeable the membrane
variables to control:
-temperature
-ethanol concentration
-No excess dye on cut sections of beetroot (rinse off)
-swirl liquid before putting in the cuvette for the
-time
-beetroot (age, same plant, same part)
-SA and/or mass of beetroot used.
** remember
judging colors by eye is not as good as using colorimeter because the results are judged subjectively or by using a colorimeter
Practical 10: Using a potometer (Factors affecting transpiration rates)
1. cut a shoot underwater to prevent air from entering the xylem (interferes with the column of wtaer travelling up the xylem) cut a slant to increase the surface area available for water uptake.
2. Assemble the potometer in the water and insert the shoot underwater, so no air can enter the water.
3. remove the apparatus from the water but keep the end of the capillary tube submerged in a beaker of water.
4. check the apparatus is watertight (vaselline etc)
5 dry the leaves to allow time for shoots to acclimatize
6. remove the end of the capillary tube from the beaker of water until one air bubble had formed, put the capillary tube back into the water.
7. record the starting position of the air bubble along the ruler.
8. start the stopwatch and record the distance moved by the bubble per unit time
9. rate of air bubble movement is the rate of transpiration
10. all other conditions must be kept constant. only one change should be variable.
independent variables:
- wind speed
- use a fan
-humidity (plastic bag over the plant)
-light intensity ( lamp distance away from plant)
-temperature
variables to control
water uptake
no all water is ivolved in transpiration
some may be used to maintain tugidity or some use in photosynthesis
Practical 13: Water potential of potato (The effect of solutions of different water potentials on plant/animal cells)
1. prepare sucrose solutions at different concentrations
2. use a cork borer or chip maker to cut potatoes into same-sized pieces
3. dry the potato chips with paper towel (same method for a potato cylinders)
4. measure the mass of each potato chip and record it
5. place one potato chip in each solution
6 leave the chips in solution for the same amount of time
7. remove the chips and pat gently with a paper towel
8. re-weigh each group again and record results
9. calculate the percentage change in mass for each potato chip
10. plot results on graph
Independent variable
-Water potential of the surrounding liquid
- type of cell
variables to control
-time
-mass
-size
- surface area of tissue
- same plant/ tissue (part of plant)
- potato/ visking tubing is dry
** the points at which the graph crosses the x-axis is the isotonic point
Practical 14: Osmosis in an artificial cell
like a potato but with different water potential in artificial cell and in surrounding solutions and no need to dry the
Practical 15: Rate of diffusion through a membrane (Factors aff ecting diffusion in model cells)
1. make up agar jelly with phenolpthalein and dilute sodium hydroxide
2. fill a beaker with some dilute hydrochloric acid
3. cut out cubes of different dimensions
4. time how long it take for cubes to go colourless
5. calculate the rate at which it took for the colour to disappear
OR
cut cubes the same size but submerge in different concentrations of HCl
variables to control
- temperature
-size and shape of agar blocks
Practical 16: Qualitative testing for proteins
1. add a few drop sodium hydroxide solution
2. add equal volume of biuret solution
if protein is present the solution will turn from blue to purple
Practical 17: qualitative testing for lipids )Emulsion test
1. Add equal volume ethanol
2. shake the solution
3. add solution into distille water
if lipid present their will e a milky emulsion
Practical 18: Qualitative testing for sugars (reducing and non-reducing)
reducing sugars (all monosaccharide and dissacharides except sucrose)
1. Add the same volume Benedict's reagent to s ample and heat it in a boiling water bath
2.if test is positive then coloured precipitate will form on spectrum blue to red
non-reducing sugars
1. add dilute hydrochloric acid
2, heat in a boiliing water bath
3.neutralise with sodium hydrogencarbonate
4. carry out benedicts test
5 if results are positive then you have a reducing sugar
Practical 19: Test for starch
1. add iodine
2. if starch is present it will turn from browny-orange to blue-black colour
Paper/Thin-Layer Chromatography
1. Grind up several leaves with anhydrous sodium sulfate and propanone.
2. Transfer the liquid to test tube, add some petroleum ether and gently shake the tube. Two distinct layers form in the liquid, the top layer is the pigments mixed in with the petroleum ether.
3. Transfer some of the liquid from the top layer into a second test tube with some anhydrous sodium sulfate.
4. Draw a horizontal pencil line near the bottom of a chromatography plate (silica plate). Build up a single concentrated spot of the liquid on the line by applying several drops and ensuring each one is dry before the next one is added. This is the point of oriigin.
5.Once the point of origin is completely dry, put the plate into a glass beaker with some prepared solvent ( eg. a mixture of propanone, cyclohexane, and petroleum ether. Lower is just enough so that the point of origin is slightly above the solvent. Put a lid on the beaker and leave the plate to develop. As the solvent spreads up the plate, the different pigments move with it, but at different rates - so they separate.
6.When the solvent has nearly reached the top, take the plate out and mark the solvent front (the furthest point the solvent has reached) with a pencil before it evaporates and leave the plate to dry in a well-ventilated place.
7. There should be several new coloured spots on the chromatography plate between the point of origin and the solvent front. These are the separated pigments. You can calculate the rF values and look them up in a data base to identify the pigments.
DNA extraction
grind a sample in pestle and mortar
mix detergent with the sample - breaks down the cell membrane
add salt - break hydrogen bonds
add protease enzymes- brekadown histone proteins
add ethanol cause dna to precipitate out of the solution
DNA will be seen as white strands forming bewteen the layer of sample and layer of alcohol
condensation reaction
mono-> poly, water molecule released, new covalent bond formed
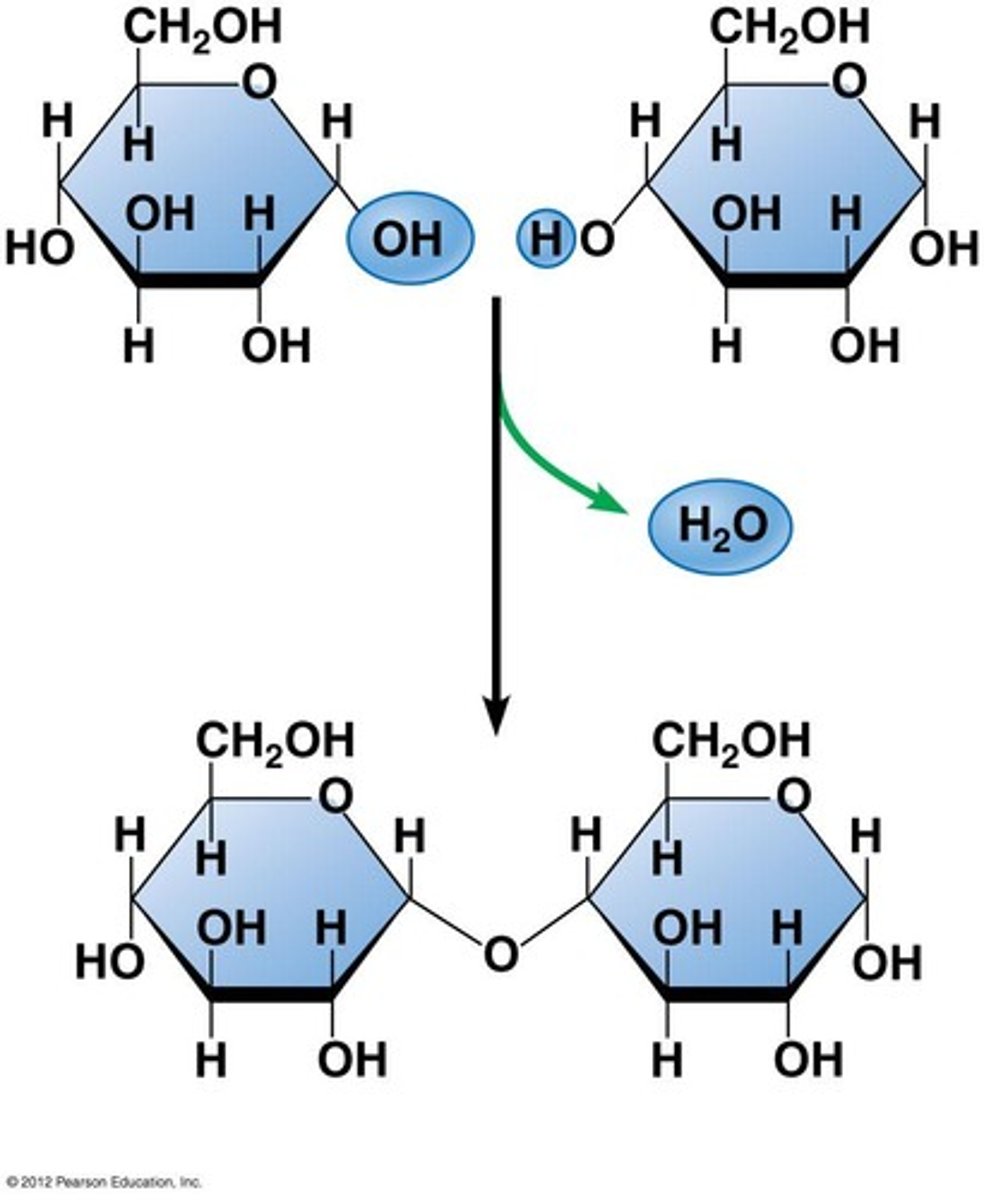
hydrolysis reaction
poly-> mono, water molecule used, covalent bond broken
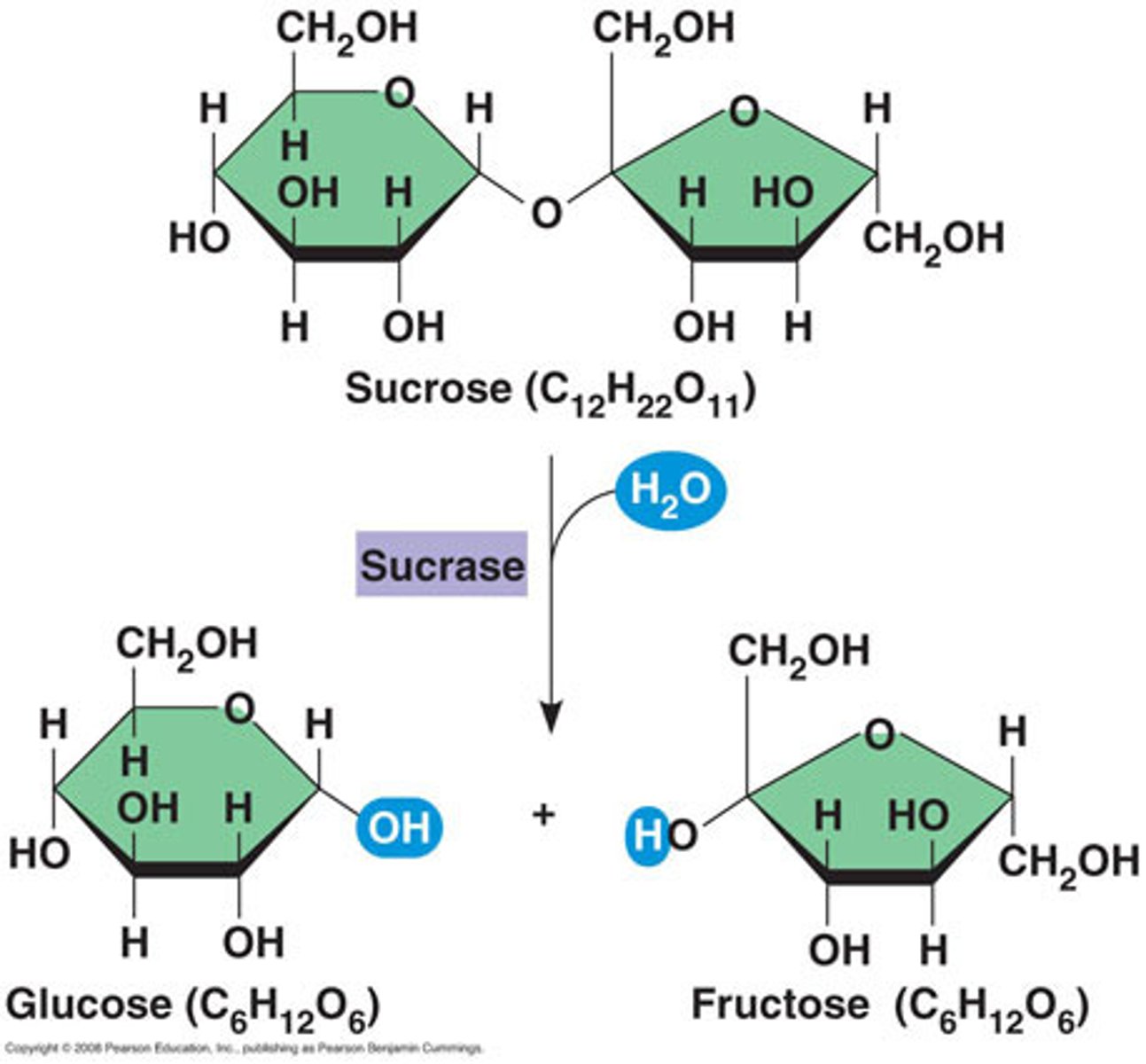
functions of carbohydrates
energy source- glucose.
energy store- starch, glycogen.
structure- cellulose.
form parts of large molecules ie nucleic acids, glycolipids/proteins
monosaccharides
simple sugars eg glucose, fructose, galactose, ribose
disaccharides
'double sugars' eg maltose, sucrose, lactose
polysaccharides
large molecules eg starch, glycogen, cellulose
Types of monosaccharides
Triose (3 carbons) Pentose (5 carbons) Hexose (6 carbons)
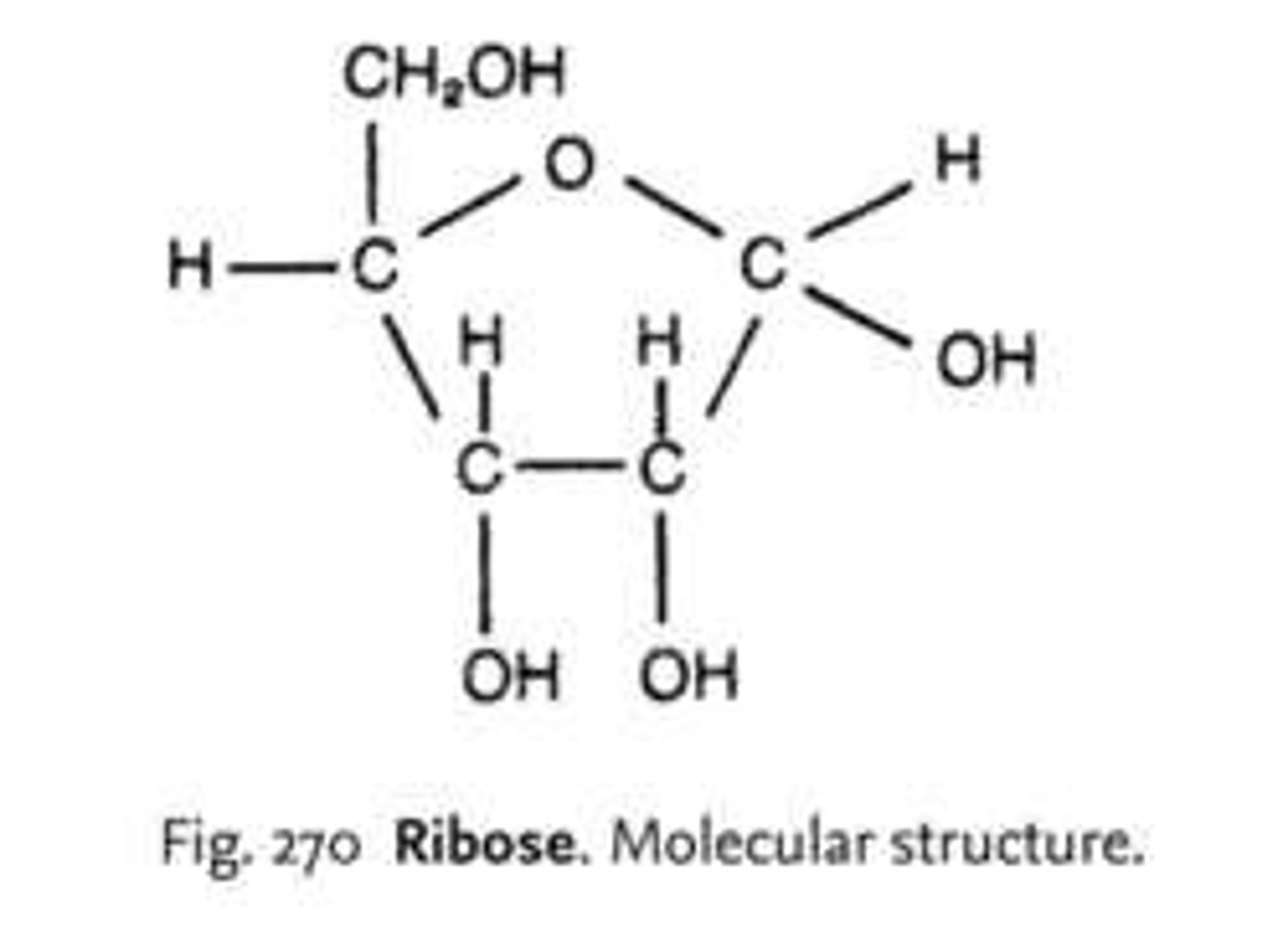
ribose
C₅H₁₀O₅, found in RNA, pentose sugar.
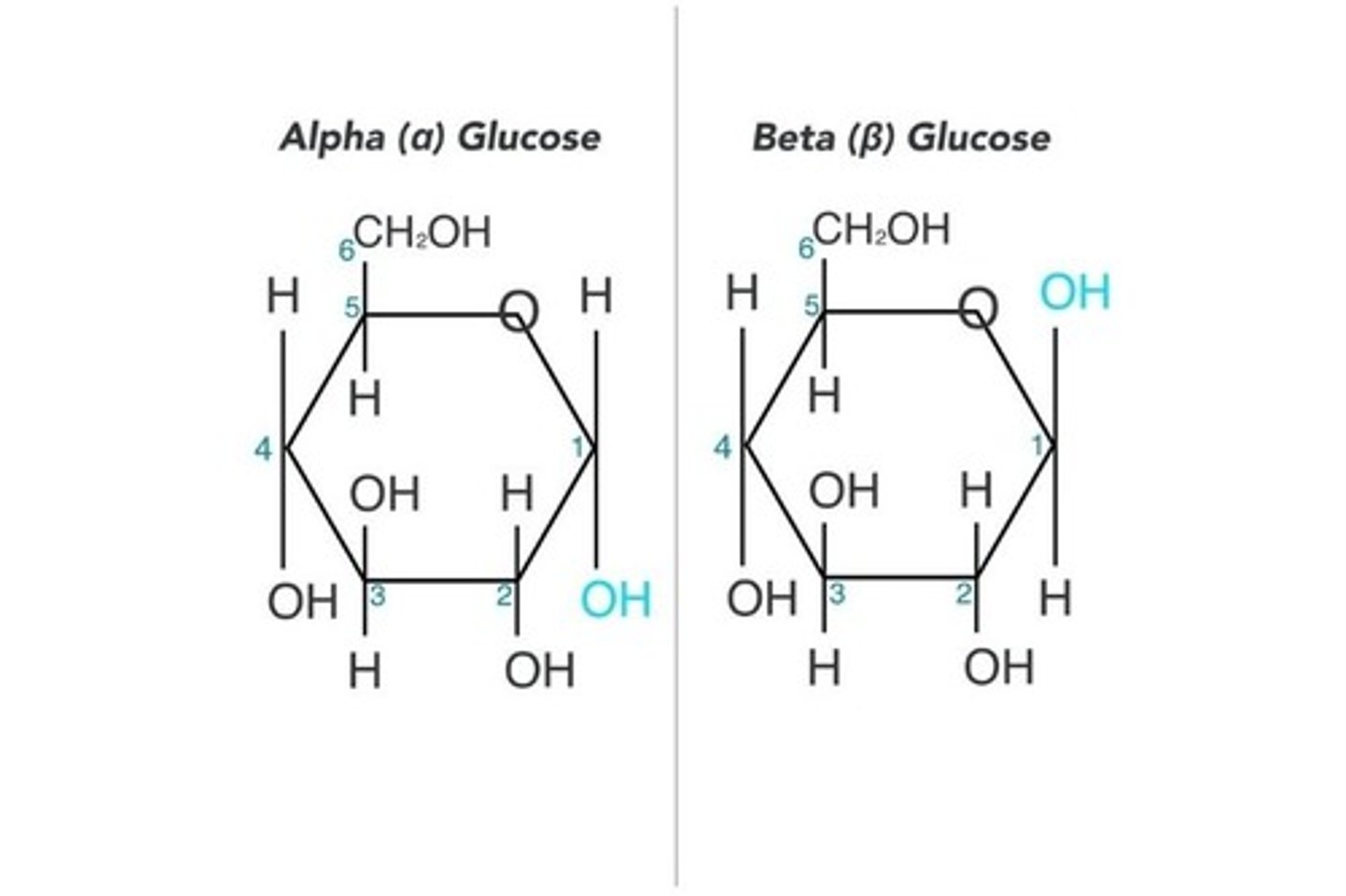
glucose
C₆H₁₂O₆, major energy source, highly soluble. alpha and beta glucose
galactose
C₆H₁₂O₆, glucose isomer. not as soluble, important in glycolipid/protein production
fructose
C₆H₁₂O₆, glucose isomer, very soluble, main sugar in fruits and nectar- sweeter than glucose
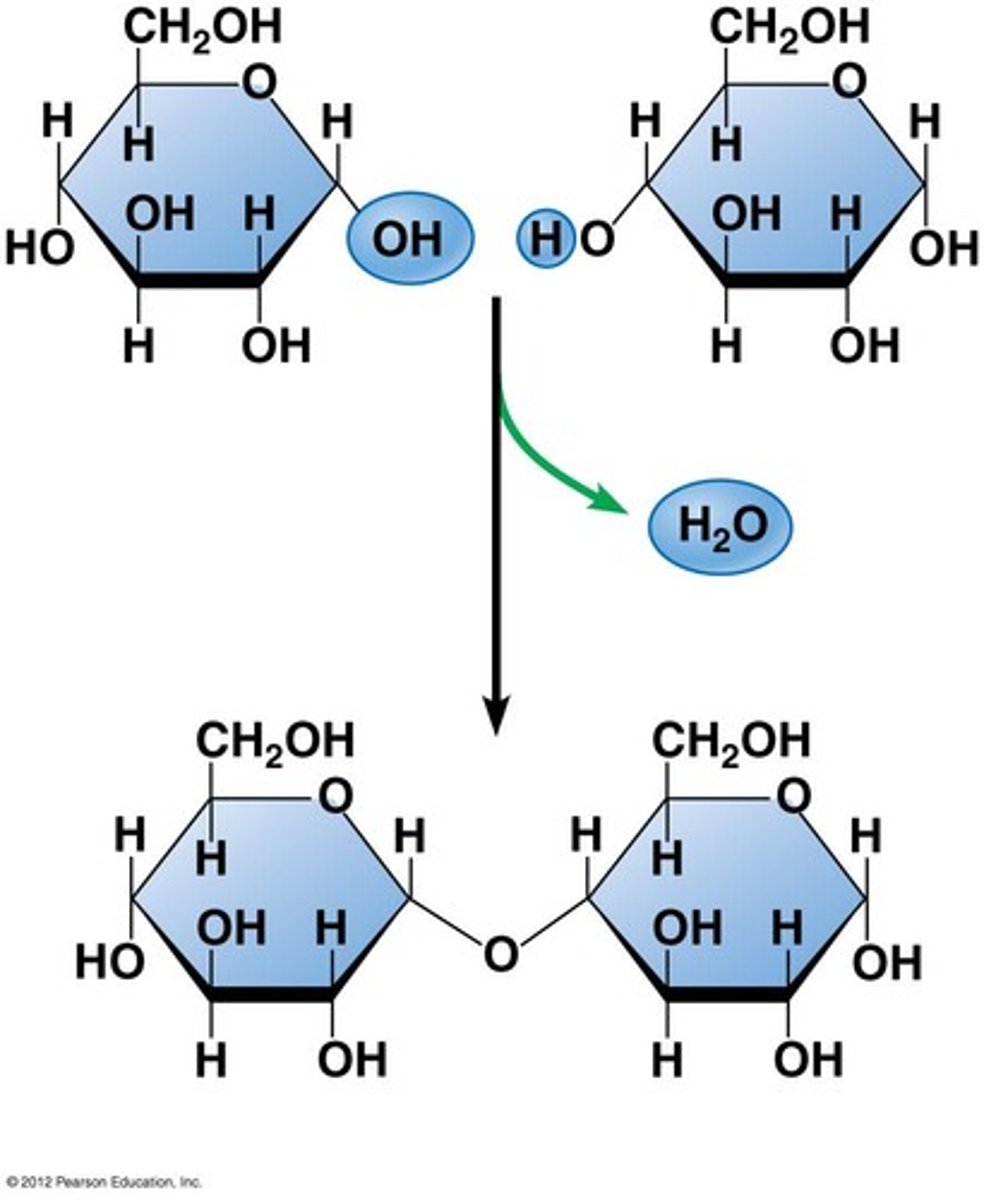
formation of disaccharides
1-4 glycosidic bond. maltose= glucose + glucose. sucrose= glucose + fructose. lactose= glucose + galactose
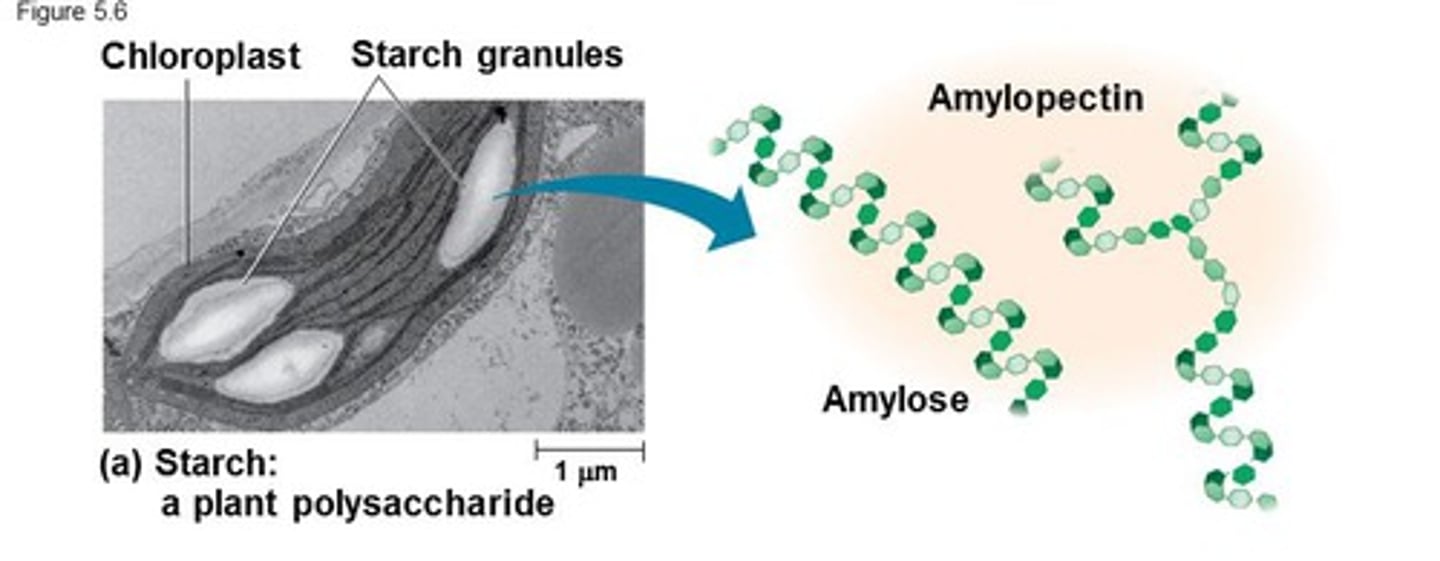
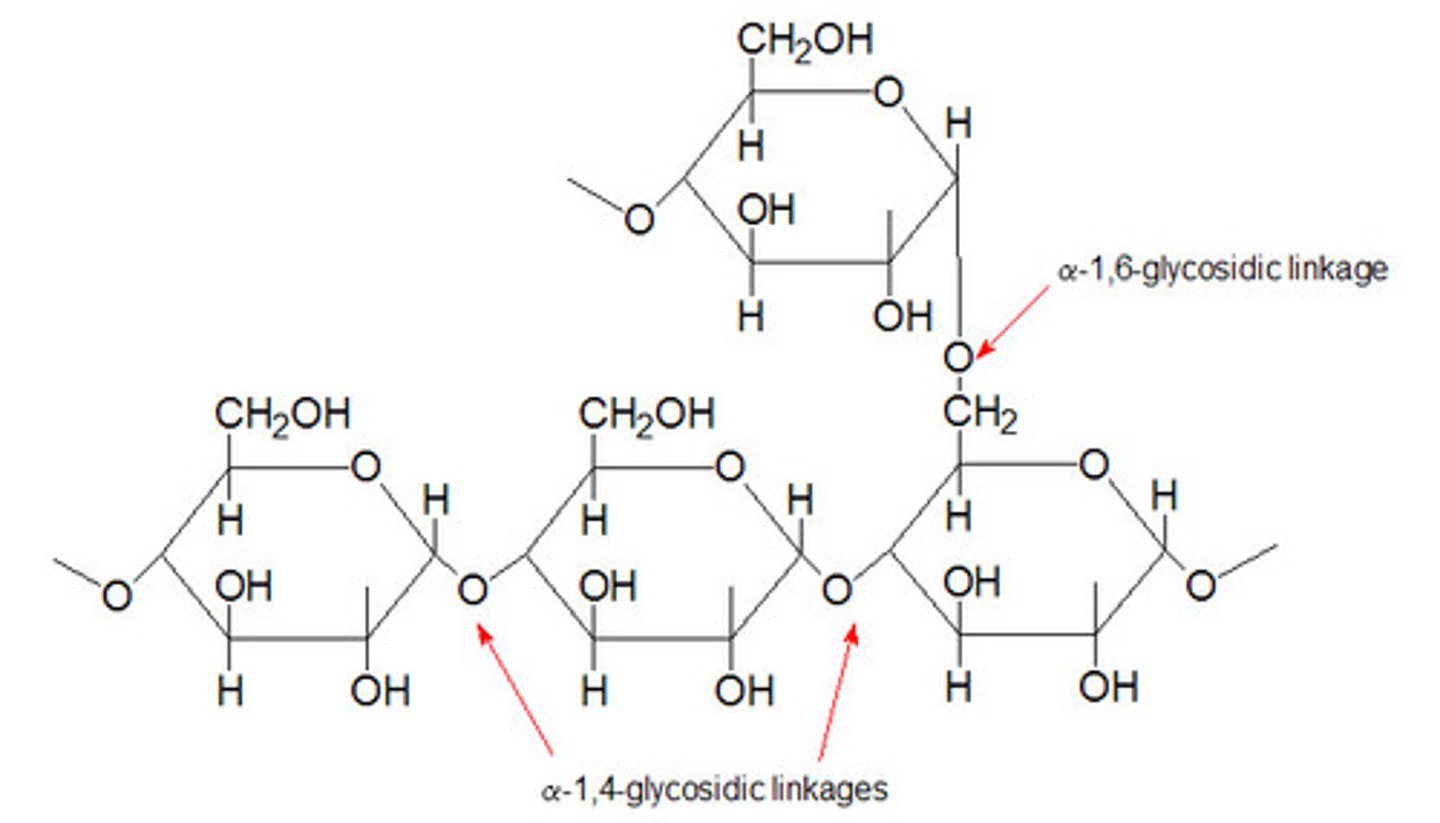
starch contains a mixture of;
amylose; 1-4 glycosidic bonds, a glucose, coiled structure. + amylopectin which has the same traits + side branches 1-6 glycosidic bonds
similarities of starch + glycogen
both energy store molecules, insoluble. more 'ends' can be quickly broken up
glycogen
1-4 glycosidic bonds, alpha glucose, coiled structure. with many side branches, 1-6 glycosidic bonds. like amylopectin but more branches- more compact, easier to break down.
cellulose
b glucose- each alternate 1 flipped- straight chain.
microfibrils
several hundered b glucose molecules cross-link via H bonds, crosslink forming macrofibrils. chains → microfibrils → macrofibrils → cellulose fibres
types of lipids
triglycerides (faits + oils) cholesterol, steroids, phospholipids.
functions of lipids
energy- fat stores long term. biological membranes, insulation ie myelin sheath, protection ie leaf cuticle, steroid hormones.
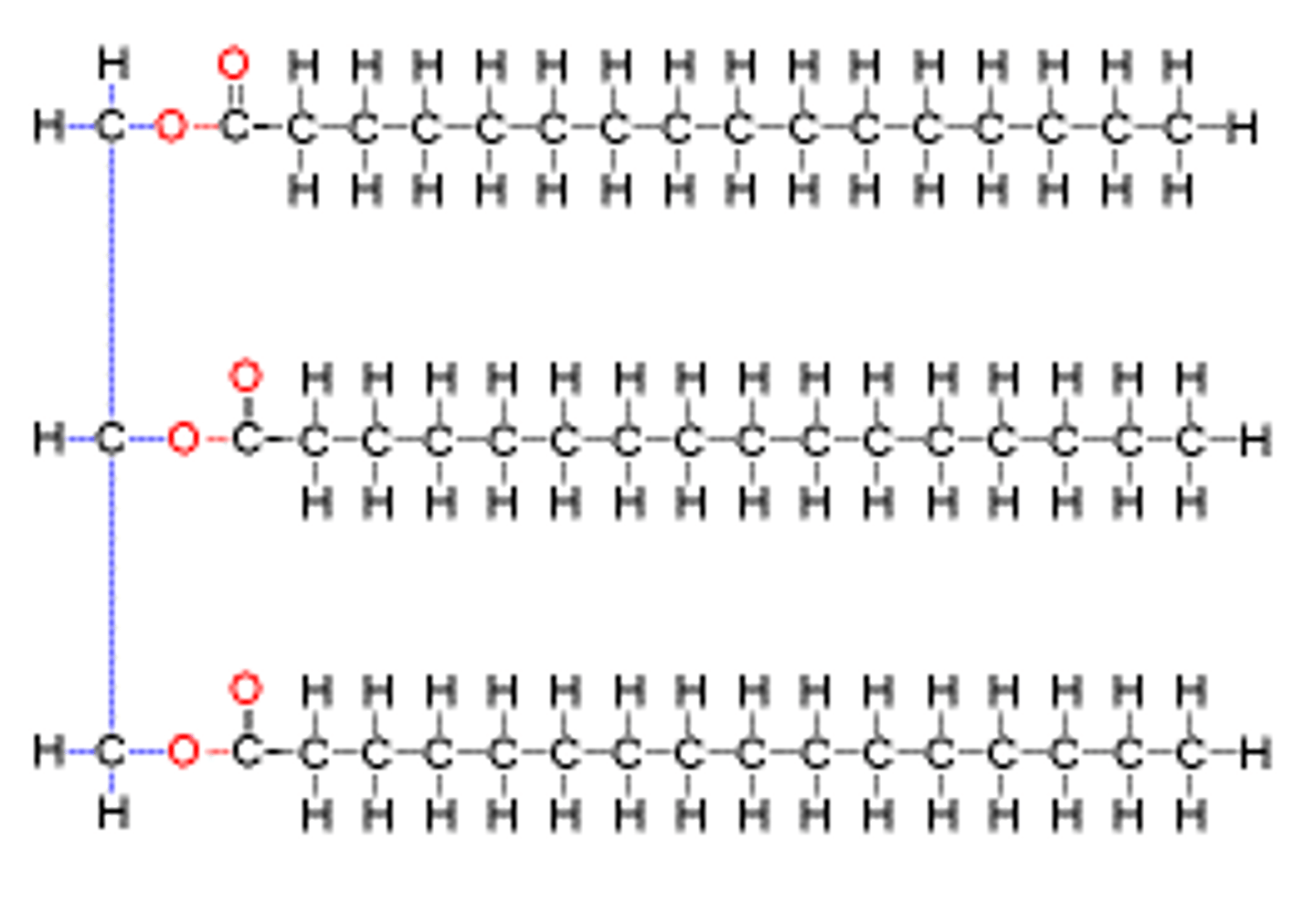
triglycerides
made from glycerol + fatty acids. COOH one end, rest hydrocarbones. unsaturated or saturated, saturated fit CₙH(₂ₙh)COOH. 15-18 carbons long
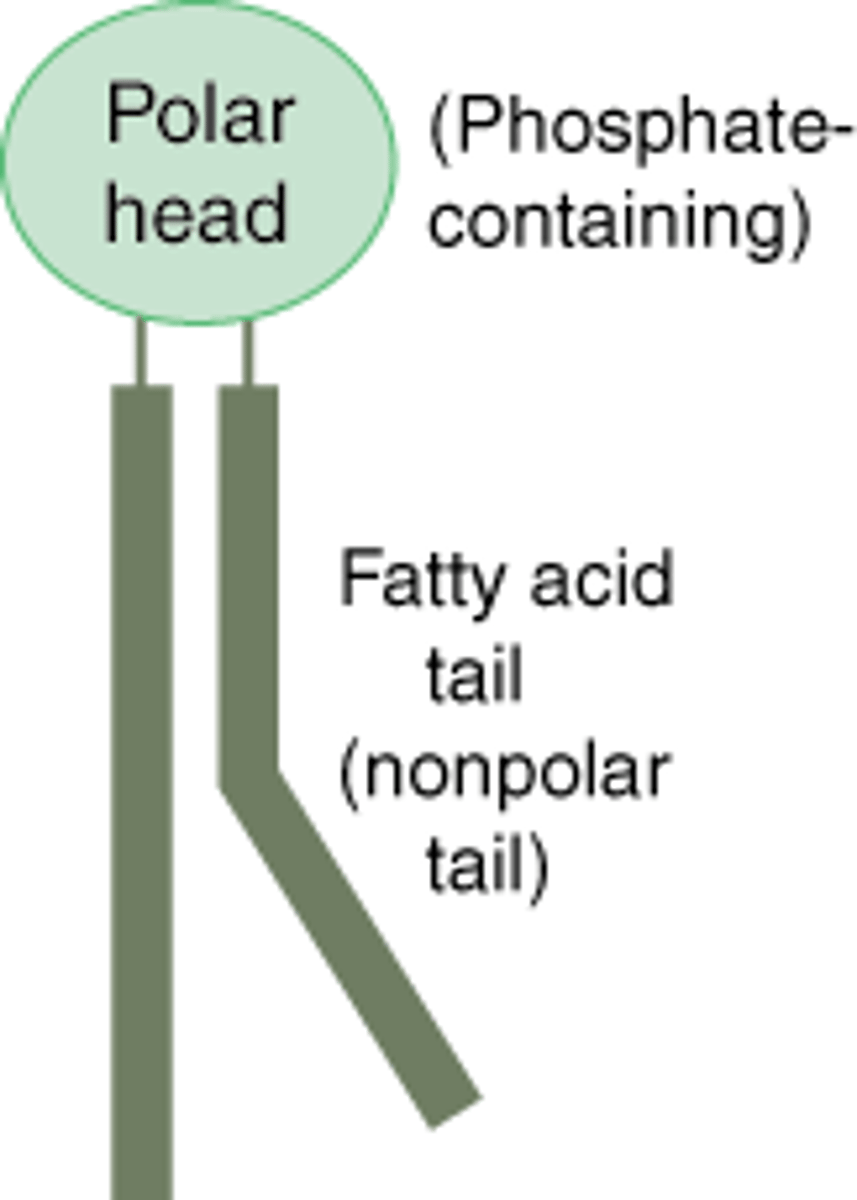
phospholipids
PO⁴⁻ hydrophilic phosphate head, hydrophobic fatty acid tails (saturated or unsaturated)
cholesterol
4c rings- small narrow molecule, hydrophobic. found in membranes fitting between tails, regulates fluidity & permeability, makes membrane more hydrophobic. make up steroid hormones in the liver ie testosterone, oestrogen, cortisol.
water
hydrogen bonds. slight - charge on the oxygen attracts slight + hydrogen atom. weak bond individually.
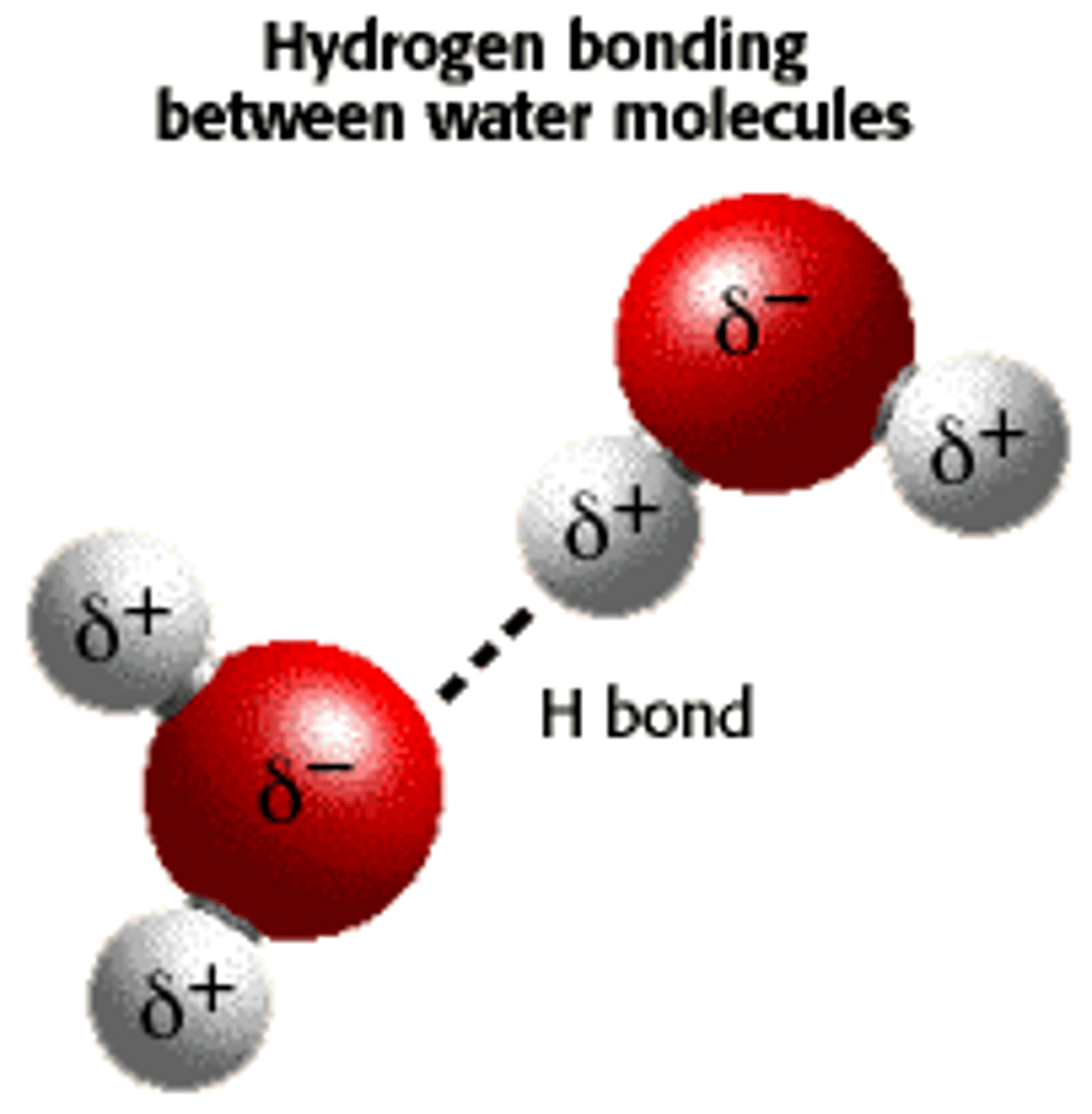
water properties
high latent heat of vaporisation- evaporation efficient cooling mechanism, ie thermoregulation.
high specific heat capacity- thermally stable aquatic environment
H bonds make ice form a lattice- ice less dense, floats, insulating water underneath
effective solvent- medium for enzyme controlled reactions, nitrates etc taken up by roots
cohesion/adhesion- water molecules cohesion helps transpiration steam
transparent- light allows underwater plants to photosynthesise
head & tail
hydrophilic phosphate head w/ amphipathic charge, hydrophobic fatty acid tail w/ equal charge
cell membrane function
controls passage, reaction site, compartmentalise, communications, binding site for hormones + drugs
intergral proteins
span whole width, many carrier or channel, transport sugars, ions, AA. some receptors for hormones, neurotransmitters, enzymes.
peripheral/ extrinsic proteins
confined to inner or outer surface. extracellular- receptor. cytosolic- reactions or cell-signalling.
glycoproteins/lipids
receptor sites
cholesterol
more cholesterol- less fluid & permeable. prevents cells from bursting at high temps.
effect of solvents on permeability
dissolve the phospholipids- more permeable
effect of temperature on permeability
low temps- crystal form piercing the membrane- increasing permeability. high temps-proteins denature, more KE so phospholipids move away from each other. H20 expands placing pressure on membrane
diffusion
down conc gradient. uses KE. sd temp, gradient and SA increase, rate increases. size increases, rate decreases.
facilitated diffusion
larger or polar molecules + ions down conc gradient. channel forms pore, carrier shaped for specific molecules, change shape permitting passage.
active transport
ions + molecules up the gradient. uses ATP + carrier protein, much faster
bulk transport
larger molecules + solid clump, ie hormones, enzymes, lipids. uses ATP. endocytosis (enter) engulf, exocytosis (exit) expel
osmosis
water from area of high water potential to low water potential across a ppm.
water potential Ψ (psi)
kPa, measure of ability of water molecules to move freely in solution. pure water is highest- 0kPA
hypotonic
higher water potential outside than inside. causes water to enter by osmosis and swell- A cell bursts, lysed, P cell vacuole swells, turgid.
hypertonic
lower water potential outside than inside. water leaves. A cell shrivels, P cell membrane pulls from wall, flaccid + plasmolysed
transmission electron microscope (TEM)
mag x500,000, res 0.05-2nm, 2D, electrons pass through
scanning electron microscope (SEM)
mag 100,000, res 5-50nm, 3D, electrons bounce off
cell membrane
hydrophilic head, hydrophobic tail. cell communication, passage of molecules in and out, antigens and receptors. hormones
nucleus
contains chromatin. pores allow passage .nucleolus makes ribosomes.
ER
system of membranes.
R- covered in ribosomes, folds and processes proteins made
S- no ribosomes, synthesises and processes lipids
golgi apparatus
fluid filled, membrane bound. processes and packages new lipids + proteins, makes lysomes
mitochondria
cristae folded. in matrix enzymes for respiration. produces ATP. needs lots of energy
chloroplasts
membranes inside called thylakoid membranes, stacked forming grana, linked to each other by lamellae. some happens in grana, some in stroma (the thick fluid). have their own dna and ribosomes, double membrane
lysosomes
digestive enzymes- digest invading cells or break down old cell components
ribosomes
made of proteins + RNA. no membrane. makes protein
centrioles
small hollow cylinders made of microtubules. separation of chromosomes in cell division
microfilaments
narrowest protein fibers, made of actin. rigidity and movement
intermediate filaments
strands wound together. bear tension and anchor organelles
microtubules
widest. movement + resisting compression. make up cillia, undulipodia etc. vesicles move across
undulipodia
9 + 2 structure, or 2 per cell, long, unlike cilia where they are short and plentiful
role of cytoskeleton
internal framework, moving cell through a liquid or liquid past a cell, moving chromosones in mitosis, moving organelles around ie chloroplast
capillary action
adhesion- water molecules attracted to xylem's sides (the lignin)
as xylem narrow, forces of attraction pull up the water via the xylem's sides
transpiration pull
cohesion- as water molecules evaporate from leaves, water molecules are pulled up as they stick together
pull from above causes tension- cohesion-tension theory
root pressure
as minerals move actively transported into xylem, water driven into the xylem by osmosis, forcing water up the xylem
transpiration
high Ψ due to mineral ion- water enters rootvs via osmosis. moves through roots to caspiarian strip- forced to enter cytoplasm.
xylem Ψ lowers increasing osmosis, water enters xylem moving by root pressure, capillary action or transpiration stream until spongy mesophyll of leaf- evaporates.
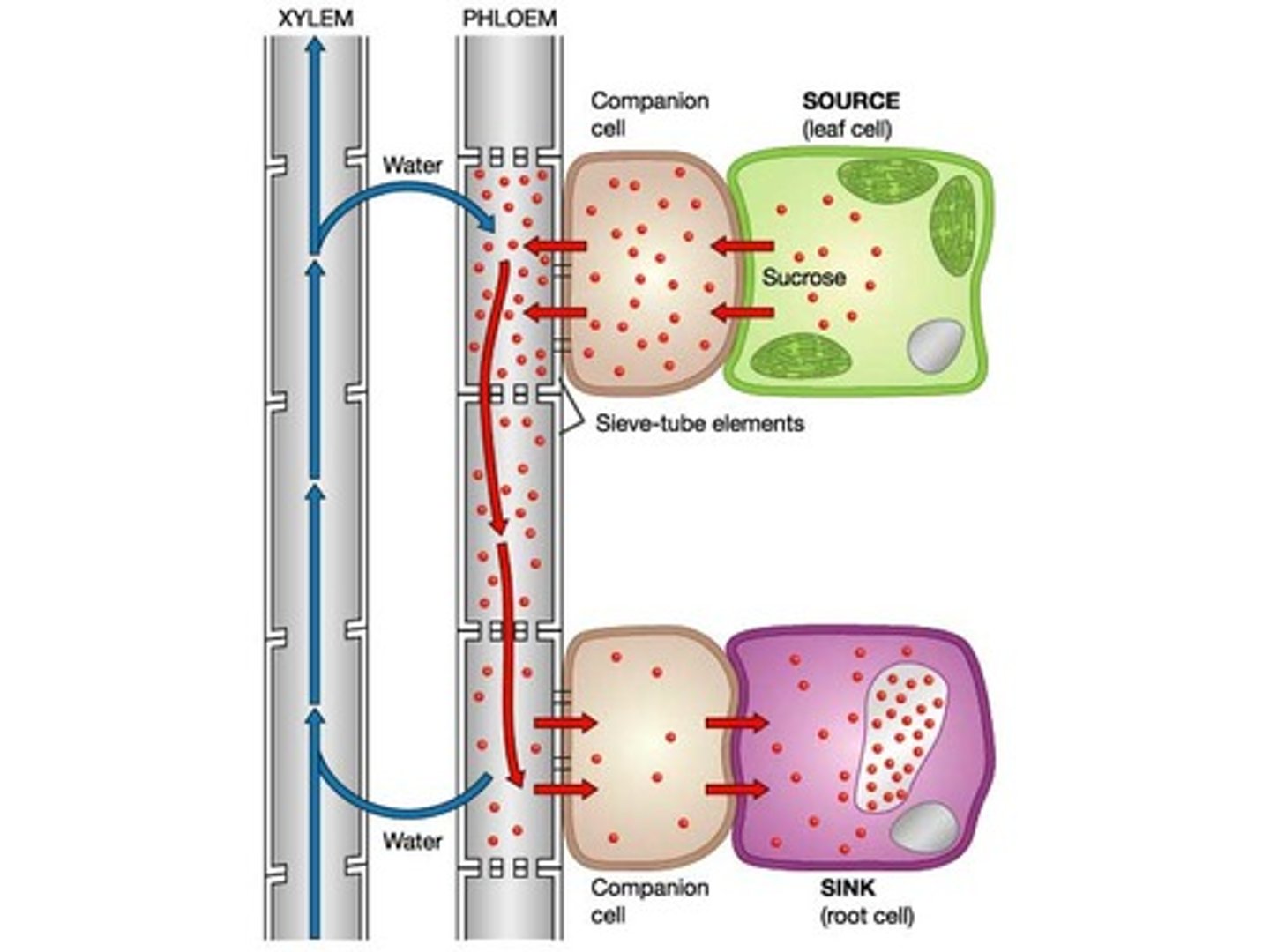
translocation
companion cells AT H+ into surrounding cells, which diffuse back bringing Na. conc of Na builds up in companion, diffusing into phloem via plasmodesmata. Na enters phloem lowering Ψ -> water enters via osmosis
cardiac cycle wordy
SAN creates electrical impulses -> atria wall -> atria systole. non contracting tissue stops spread to ventricles, AVN picks up excitation, delays 0.1s -> pass down septum via purkyne -> ventricle walls -> ventricular systole
cardiac cycle simplified
SAN -> AVM -> purkyne fibres
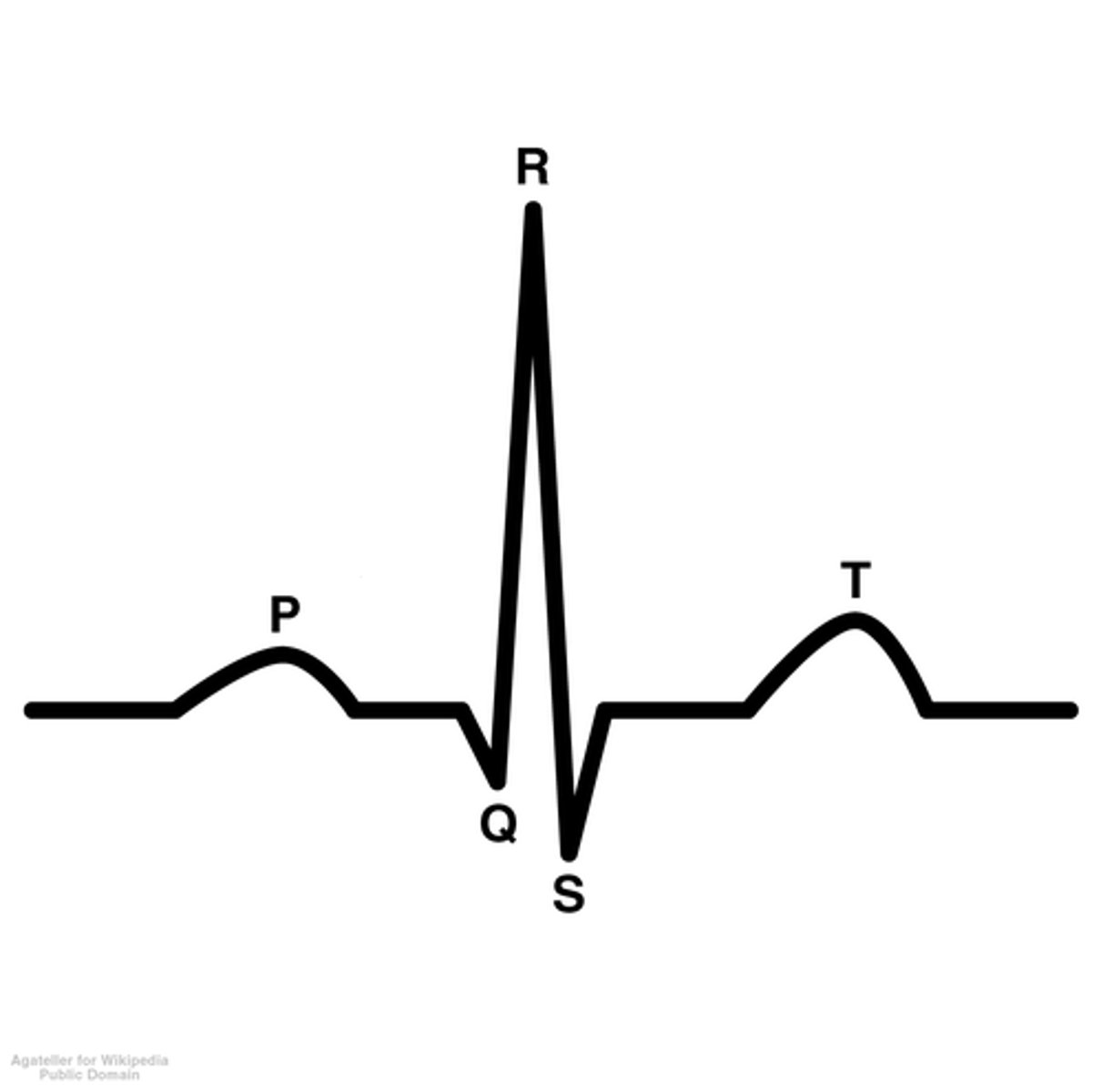
PQRST
P- atrial systole
QRS complex- ventricular systole
T- diastole
breathing in
diaphgram contracts, moves down
ex icm contract moving rips up & out
in icm relax
chest vol increase
pressure falls below atmospheric
breathing out
diaphragm relaxes, moves up
ex icm relax moving ribs down & in
in icm contract
chest vol decreases
pressure rises above atmoshperic
ventilation in fish
open mouth lowering floor of buccal cavity increasing volume decreasing pressure, water enters. fish closes mouth, volume decreases and pressure increases forcing water out across gill filaments
ventilation in insects
air moves into spiracles then tiny air filled tracheae, branching into tracheoles w thin permeable walls, then cells. oxygen diffuses this way, co2 the other way
rhythmic abdominal movements and wing movements move air in and out of the spiracles
respiration- 1. glycolysis
1. phosphorylation- glucose -> hexose -> hexose biphos -> 2 TP
2. oxidation- TP -> 2 pryuvate + 2 NADH
respiration- 2. link reaction
pryuvate ATed to mitochondrial matrix, decarb, NAD-> NADH dehy pryuvates H-> acetate. + coA -> acetyl coA
respiration- 3. krebs
acetyl coA joins 4C-> citrate, decarb+dehy-> 5c compound, this hydrogen produces red NAD+FAD. further decarb+dehy, now oxaloacetate
respiration- 4. oxidative phosphorylation
red NAD+FAD oxidised, H splits to H+ & e-. e- move along ETC pumping protons into intermembrane space-> electrochemical gradient, protons move down via atp synthase, driving atp synthesis- chemoismosis.
protons, electrons + O2 from blood combine forming water at end
photosynthesis- 1. cyclic phosphorylation
photon of light hits PS1 exciting 2e-, passed along ETC making small vol of ATP via phosphorylation, electrons passed back to PS1
photosynthesis- 2. noncyclic phosphorylation
photon of light hits PS2, exciting 2e- leaving primary pigment, replaced by 2e- from photolysis, pass along ETC making small vol of ATP via chemiosmosis, 2e- accepted by PS1, which
photon of light hits exciting 2e-, leave PS1 w/ 2H+ from photolysis, join NAPD-> NAPDH.
2e- from PS2 replace those lost from PS1.
photosynthesis- 3. calvin cycle
CO2 diffuse into intercellular space+ RuPB catalysed by RuBisCo -> 6C intermediate -> 2 3C GP w/ H + ATP -> 2 3C TP. majority tecycled to RuBP (10TP -> 6RuBP)
detoxification of alcohol
ethanol -> ethanal (+2NADH) -> acetic acid (+2NADH)-> acetate
1. ultrafiltration
blood flows through glomerulus surrounding bowman's, high hydrostatic press forces small molecules to enter B's, this filtrate moves to PCT for reabsorption
2. selective reabsorption
Na/K pump reduces Na conc in cells lining PCT- Na f diff into cell w/ glucose or AAs, increasing glucose + AA conc inside, diffuse into capillary. Na, glucose + AAs reabsorbed reducing Ψ in cell - water reabsorbed by osmosis
3. osmoregulation
ascending limb ATs Na+ Cl out filtrate into tf, water stays as wall impermeable- solute conc in tf increases.
descending permeable- water osmosis out, solute conc in filtrate decreases.
bottom of ascending Na+Cl permeable- f diff out, as filtrate moves up ascending, solute conc decrease, Na+Cl ATed to tf. solute conc in medulla highest at loop base
action potential
threshold- more Na+ open, move in (depolarisation) until +40mv- Na close, K open, move out (repolarisation) everything in wrong place (hyperpolarisation) Na/K pumps re-establish resting potential (refractory period)
non-steroid hormones intercellular effect
hormone 2st messenger, binds to receptor activating G protein, activating adenyl cyclase converting ATP -> cAMP (2nd messenger) which can act directly on another protein or initiate cascade of reactions
sliding filament model
at rest myosin heads attached to ADP- action potential releases Ca from sarcoplasmic rectilium, binding to troponin- tropomyosin moved away from A-M binding site- myosin binds- ADP+Pi released -> myosin power stroke pulling actin fillaments along myosin. new ATP binds to myosin breaking A-M bridge, ATP hydrolysed.
low oxygen
detected by chemoreceptor -> sensory neurone -> cardiovascular center -> parasympathetic neurone uses noradrenaline -> SAN -> increase heart rate
low blood pressure
detected by pressure receptor -> sensory neurone -> cardiovascular center -> sympathetic neurone uses noradrenaline -> SAN chnages rhythm until increases heart rate
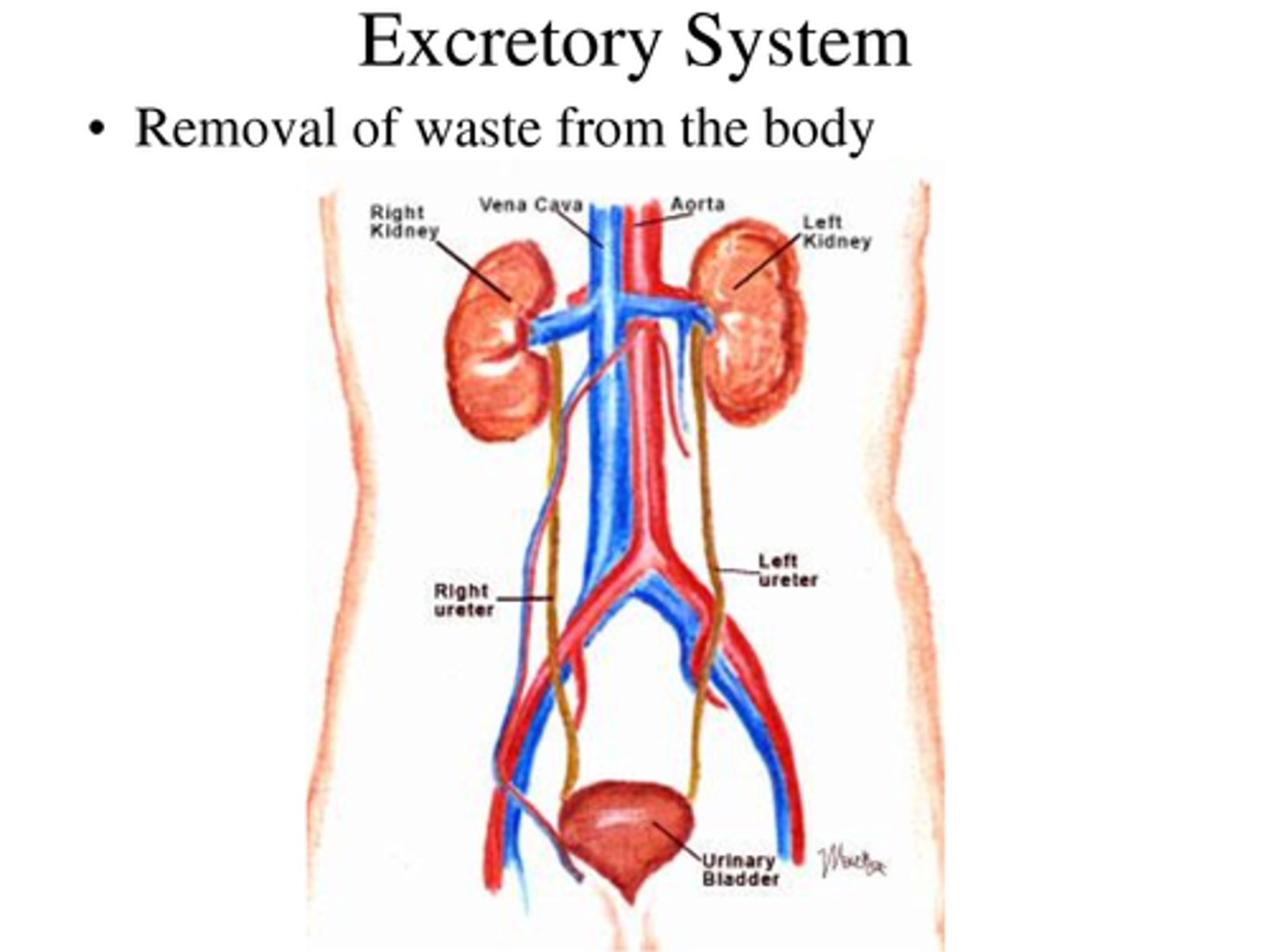
Excretion
The removal of waste products of metabolism from the body