Level 1 Mastery Check - Unit 1 🧪🧬
1/45
Earn XP
Description and Tags
skkskskkksksksksk ❤️❤️
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
What is matter?
Anything that has mass and takes up space
How do we describe matter?
With physical and chemical properties
A _ _ _ _ _ _ _ _ property can be observed without altering the identity of the substance.
Physical
Give some examples of physical properties
Color, Density, Texture, Temp, Length, Weight, Mass, Volume, etc.
An _ _ _ _ _ _ _ _ _ property is a property that changes when the size of the sample changes. Examples are mass, volume, and length
extensive
A _ _ _ _ _ _ _ _ _ property is a property that doesn’t change when you take away some of the sample. Ex: Temp, color, and hardness
intensive
Is color a intensive or extensive property?
Intensive
Is density a intensive or extensive property?
Intensive
Is texture a intensive or extensive property?
Intensive
Is temp a intensive or extensive property?
Intensive
Is length a intensive or extensive property?
Extensive
Is volume a intensive or extensive property?
Extensive
Is weight a intensive or extensive property?
Extensive
A chemical change can’t be observed without _______ the identity of the substance
altering
Give an example of some physical changes
Tearing, Crushing, Cutting, Dissolving, Phase Changing (Melting, Freezing, etc.)
Give an example of some chemical changes
Rusting, Burning, Fermenting, Metabolism
There are FIVE signs of chemical changes. Name them.
1 Odor being given off. 2 Color Change. 3 Temperature Change. 4 Formation of gas. 5 Formation of a precipitation.
Paper Burning is a _________ change
chemical
A lake freezing is a _________ change
physical
A glass breaking is a _________ change
physical
A cake baking is a _________ change
chemical
Boiling water is a _________ change
physical
A newspaper yellowing is a _________ change
chemical
What is a quantitative observation?
Something with a quantity (number)
What is a qualitative observation
A quality of something
Matter can be classified as _______ geneous or _______ geneous
Heterogeneous or Homogeneous
Homogeneous means that all parts of the substance are _________. This includes elements, compounds, and solutions.
Identical
______________ means that all parts of the substance are not identical. This includes mixtures
Heterogeneous
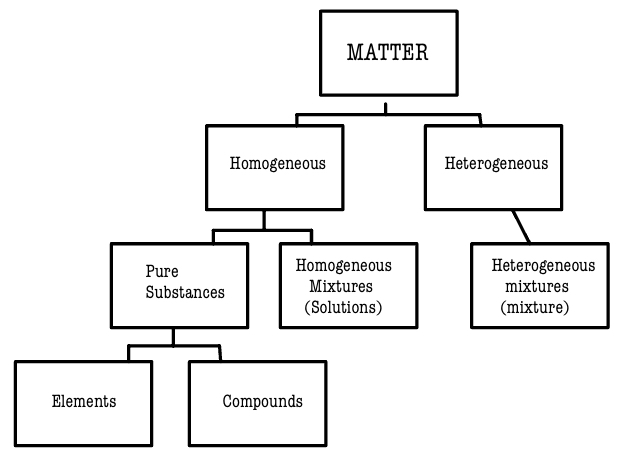
Look at this visual chart above. How does it work???
You start at matter, then see if it is homogeneous or heterogeneous and so on.
An element is the ________ pure substance
simplest
A element can’t ne changed into anything simpler through _ _ _ t or a chemical reaction
heat
An element consits of all the same _ _ _ _ s ( the building blocks of matter)
atoms
Give some examples of elements.
hydrogen, helium, carbon, nitrogen, oxygen, fluorine, neon, sodium, aluminum, silicon, chlorine, potassium, calcium, iron, nickel, copper, zinc, silver, gold, mercury (NOTE: DO NOT MEMORIZE ALL OF THESE JUST RECONIZE THEM)
_ _ _ _ _ _ _ _ s are made of more than one element; any combination of two or more different kinds of elements
compounds
Compounds are elements ___________ly combined
chemicaly
Compounds can be broken down by heating or a _ _ _ _ _ _ _ _ reaction
chemical
Compounds have a f _ _ _ _ number of components in themselves.
fixed
Give some examples of compounds.
water, carbon dioxide, carbon monoxide, sugar, hydrogen peroxide, gasoline, baking powder, vinegar, chalk, bleach, caffeine, propane, table salt, honey, aspirin (DO NO MEMORIZE; ONLY RECONIZE ALL OF THESE COMPOUNDS)
What is filtration?
Using a porous barrier to seperate a solid from a liquid in a heterogeneous mixture (a mixture)
A _ _ _ _ _ _ _ _ has two or more substances mixed together.
solution
A solution is p _ _ _ _ _ _ _ ly combined
Physically
All parts of a solution are _ _ _ _ _ _ _ throughout
uniform
Give an example of some solutions.
Coffee, Sea Water, Milk, (basically anything that is homogeneous no matter where you look at it)
A mixture is similar to a solution, except all parts are ____ __________
not identical
A mixture can be seperated by f _ _ _ _ _ _ _ _ n
filtration
Give an example of a mixture.
Soil, Pizza, Sand + Water, Cereal, etc.