BIO TOPIC 6 TO REMEMBER
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
Short tandem repeats (STRs)
Non-coding DNA contains many short, repeated sequences, usually 3-7 bases long and repeated from a few to many times
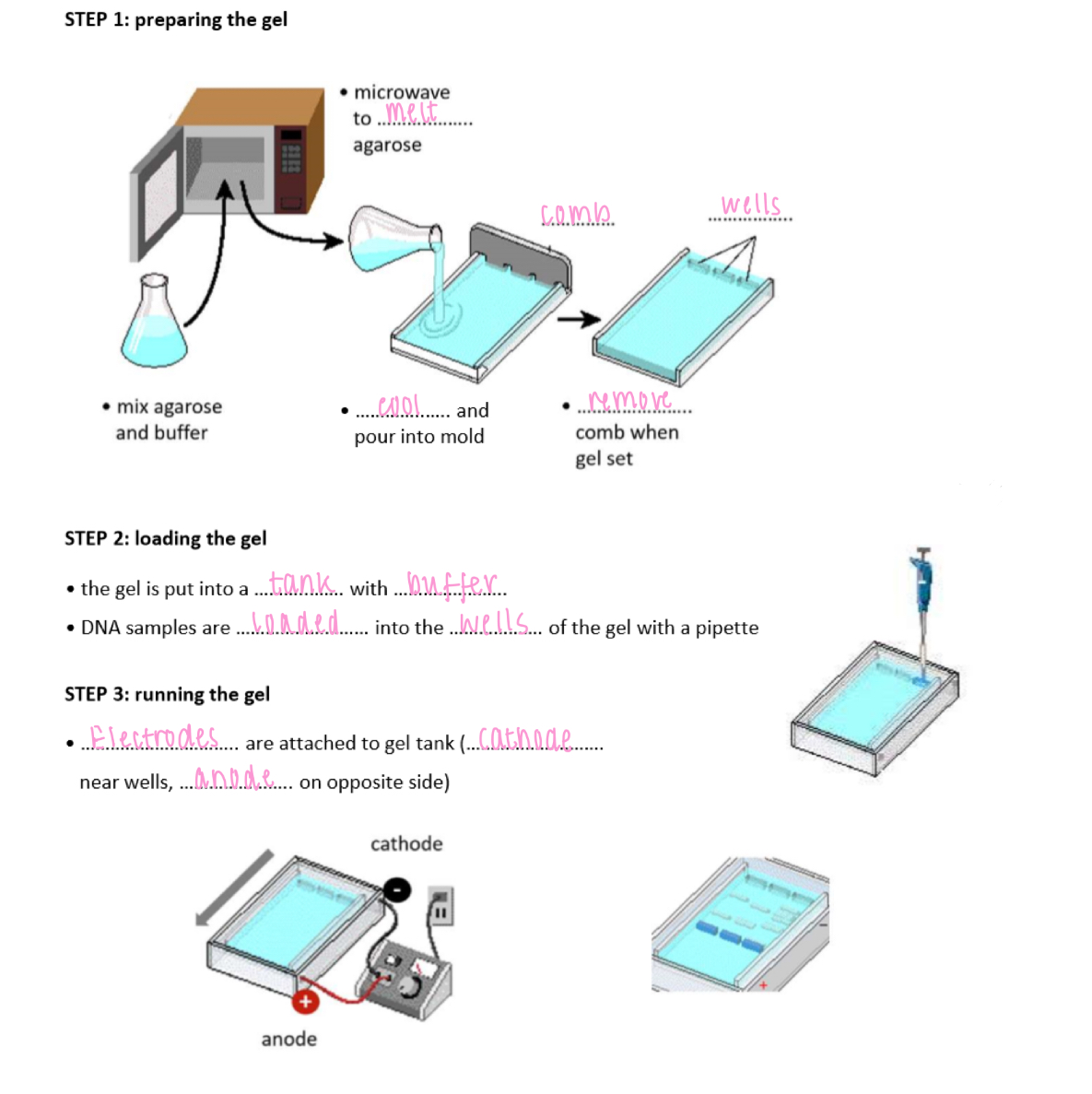
Gel electrophoresis steps
1. prepare the gel
2. load the gel
- pour the gel into the tank w/ buffer
- DNA samples are loaded into the wells of the gel w/ a pipette
3. running the gel
- electrodes are attached to the gel tank (cathode = near wells, anode = opposite side)
What's used to estimate time of death
1. Extent of decomposition
2. Stage of succession
3. Forensic entomology
4. Body temperature of the deceased
5. The degree of muscle contraction
Five stages of decomposition
1. fresh (initial decay)
2. bloating (putrefaction)
3. active decay
4. advanced decay
5. dry remains
5. Dry remains
50 to 365 days
- soft tissue lost, leaving skin, bone and cartilage
Structure of virus
Nucleic acid: either DNA or RNA, single or double-stranded
Capsid: protein coat
Some contain an envelope, attachment proteins
None contain a plasma membrane, cytoplasm or ribosome
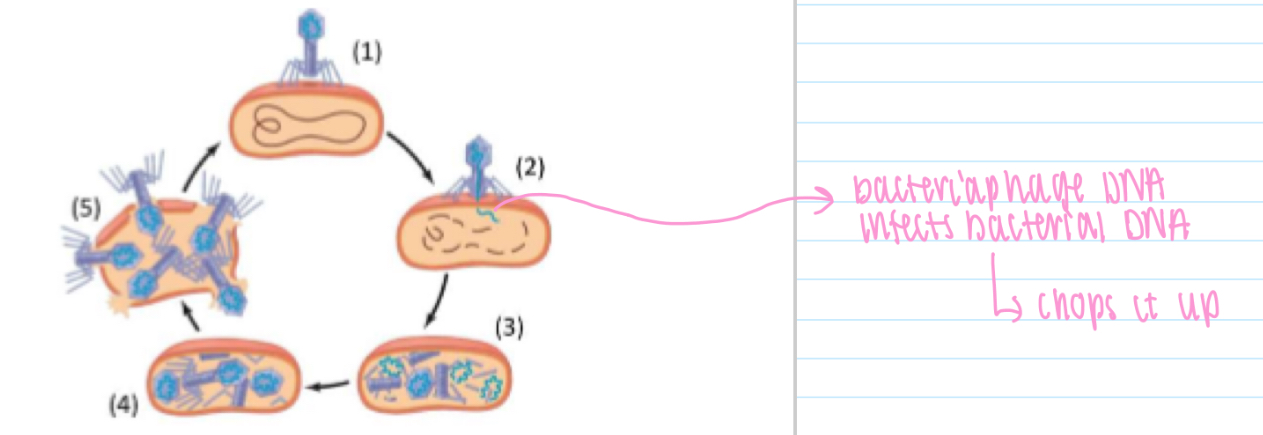
Bacteriophage life cycle
1. Attach to host bacterium
2. Phage DNA enters and destroys bacterial DNA
(3) Viral genome and proteins synthesised
(4) New bacteriophages assembled.
(5) Bacteriophages released as cell lyses (splits open)
Non specific IS - anatomical, biological factors
Gut: microbiome
Skin: microbiome
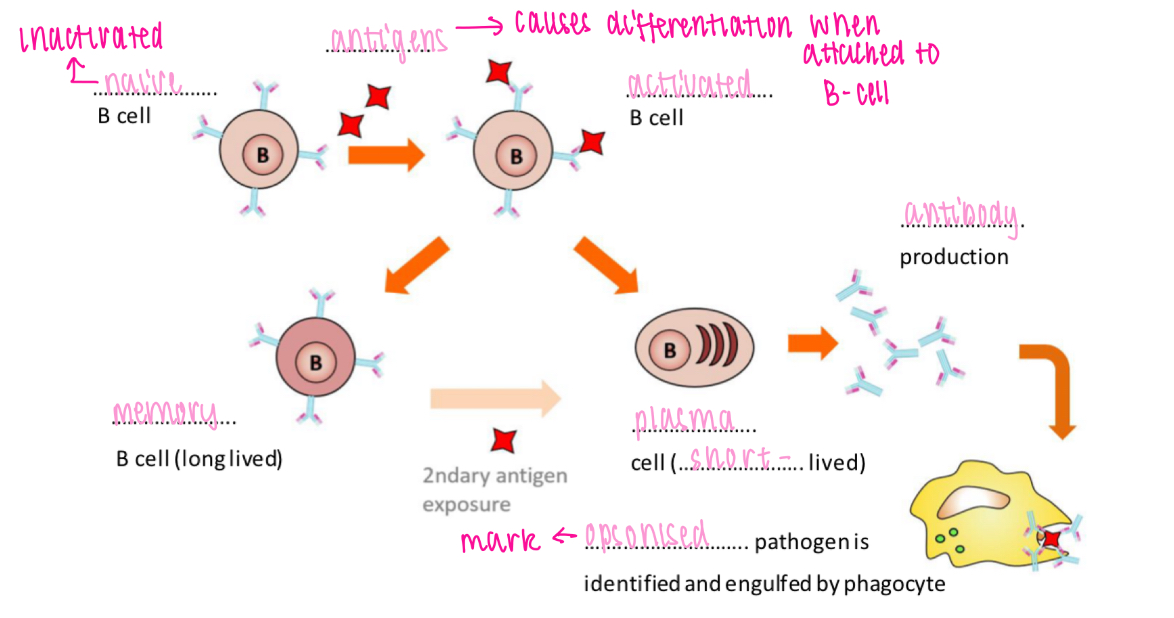
B cell receptor
Membrane-bound version of antibody
Has signal transduction functional area
Recognises antigen & initiates response in B cell
B cell activation - T cell-independent
Antigen binding triggers development of memory + plasma cells
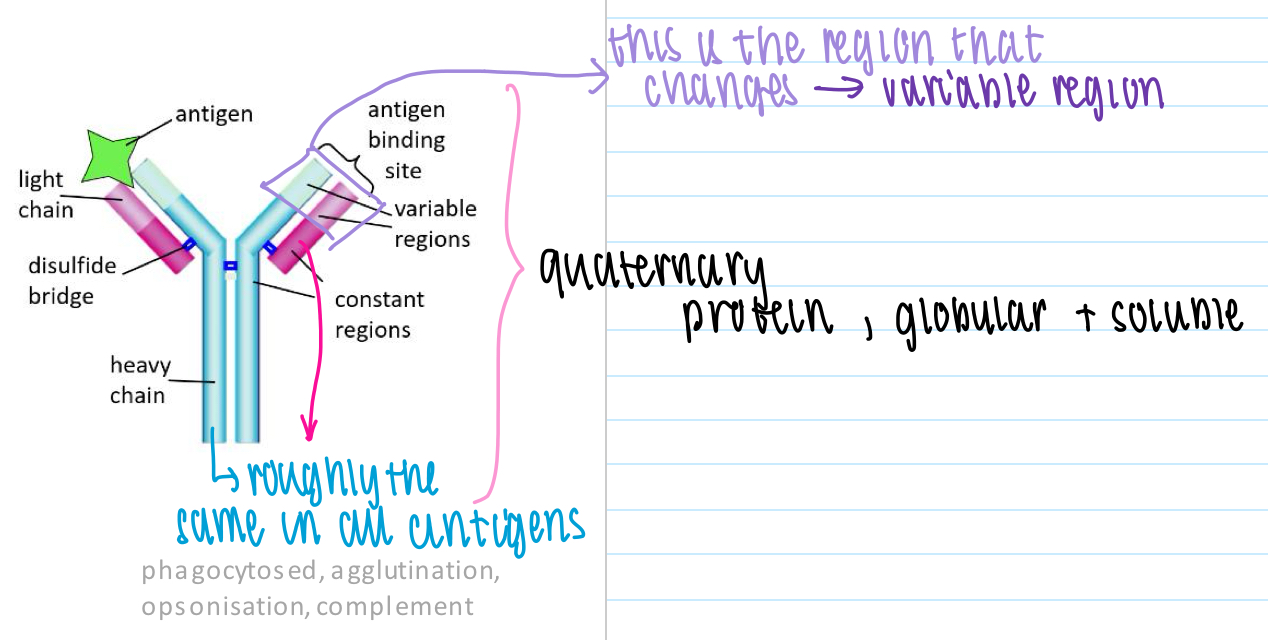
Antibody structure
2 identical light pp chains + 2 identical heavy pp chains held together by disulphide bridges
- constant regions + variable regions (for antigen binding)
- each antibody specific for one antigen
Mycobacterium tuberculosis - transmission
Only transmitted by people with active TB
Spreads person to person via coughing, sneezing, etc.
Inhaled bacteria reaching lungs may cause infection
Highly contagious, only a few bacteria (e.g. 10) needed
HIV - latent phase
12 weeks to several years:
- provirus (viral DNA) dormant in Th cell genome
- virus reproduces slowly
- IS controls infection
- no symptoms but increasing tendency to suffer infections
- possible reactivation of dormant diseases (e.g. TB)
W/O ART drugs: develops into AIDS within several years
W/ ART drugs: possible to have normal life span with HIV
Bactericidal
Kill bacteria via cell lysis
- target bacterial cell wall (e.g. penicillin)
- target bacterial cell membrane
- interfere with enzyme activity
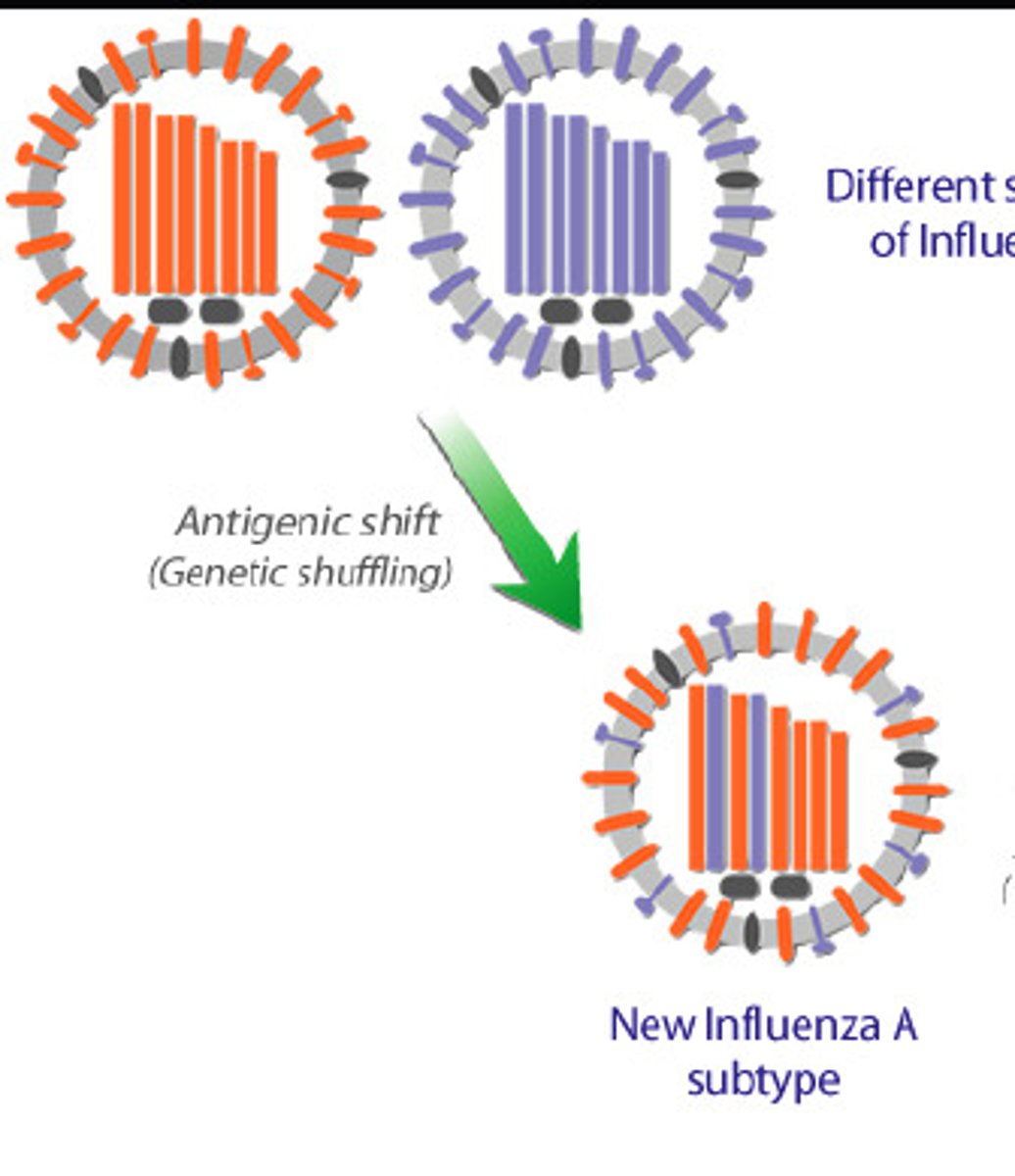
Strategy to beat IS - antigenic shift
Changes in antigens due to mixing of antigens from several species
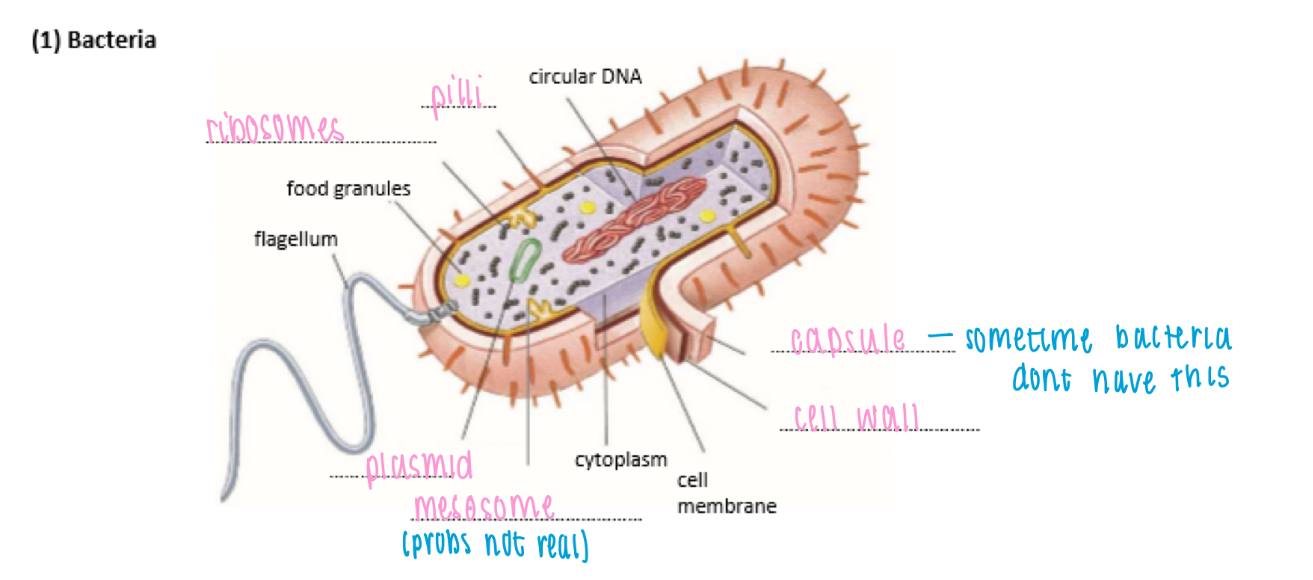
Structure of bacteria
Circular DNA: genetic code
Plasmids: small loop of DNA
Food granules: glycogen granules, lipid droplets
Mesosomes: infolding of cell membrane
Cell wall: made of peptidoglycan
Capsule: slime layer on surface for protection & to prevent dehydration
Pilli: thin protein tubes, allow bacteria to adhere to surface
Flagellum: hollow cylindrical tail-like structure, rotates to move cell
Ribosome: 70s
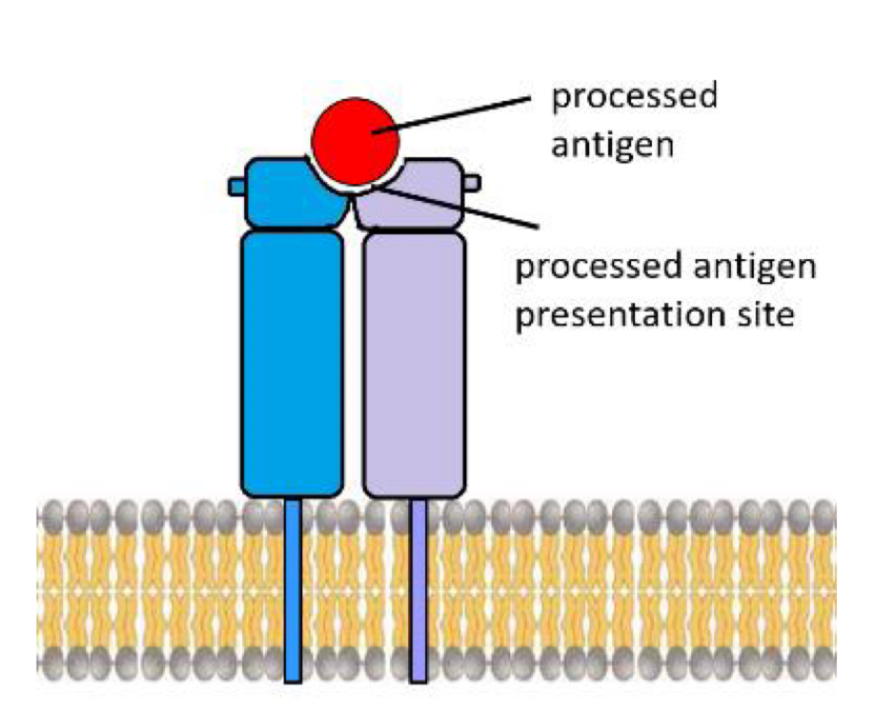
How are antigens presented
Presented on a major histocompatibility complex (MHC) on cell membrane
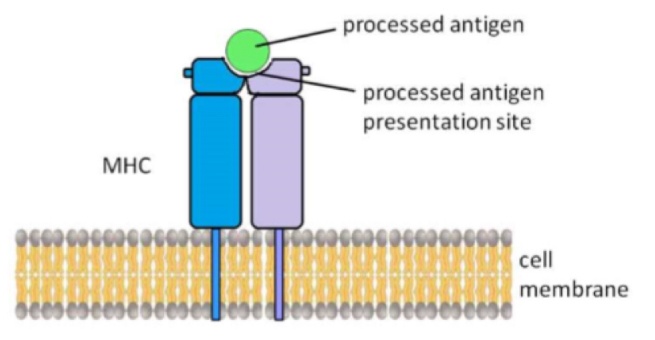
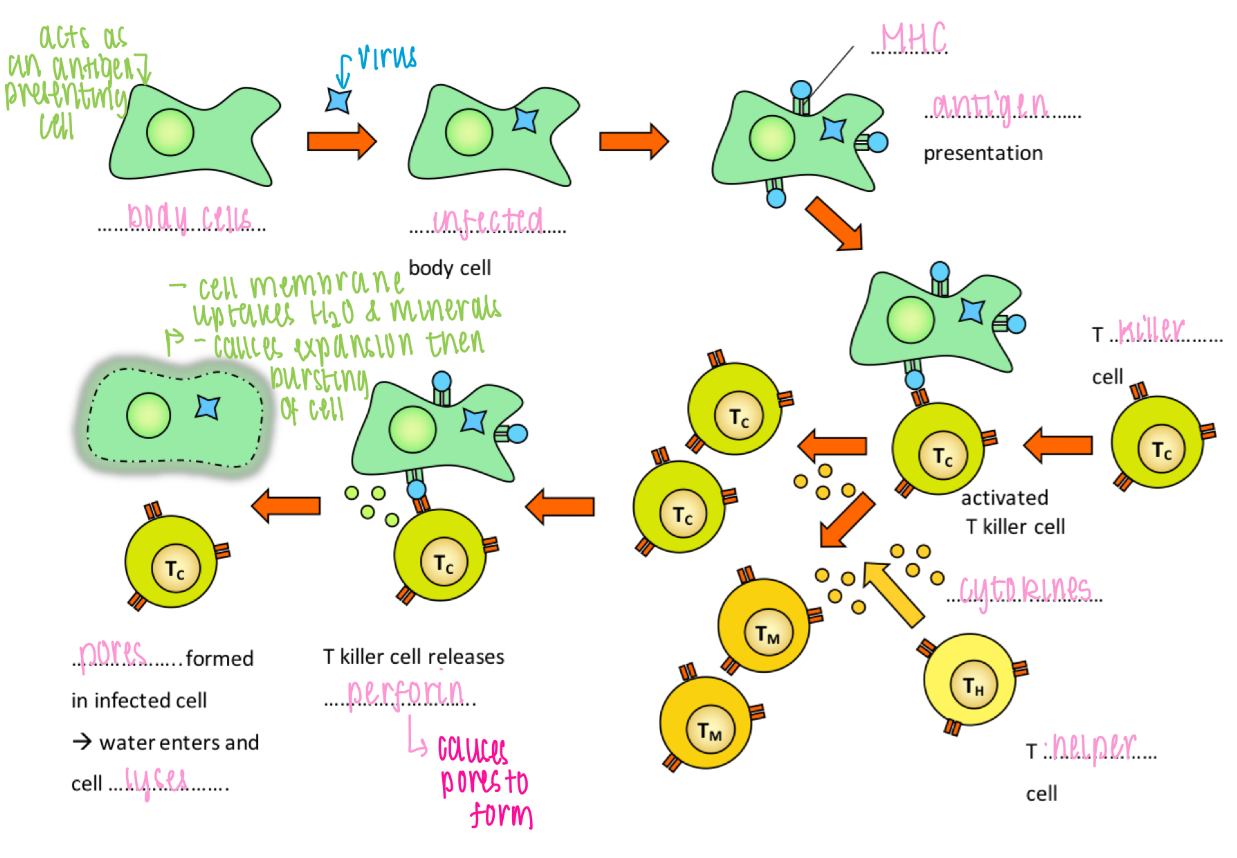
Role of T killer cells
Destruction of virus-infected cells
HIV
Human immunodeficiency virus
- targets T helper cells

Vaccine
Contains antigens (/dead/weakened pathogens) that are intentionally put into the body to induce artificial active immunity
Herd immunity
When a high enough portion of the population is vaccinated to also protect those without immunity
Vaccines for TB & HIV
TB: BCG, attenuated Mycobacterium
HIV: no vaccine because
- difficulty producing a vaccine
- virus mutates frequently giving rise to virus subtypes
Evolutionary race between pathogen and immune system
A mutation in the pathogen may help the microbe to evade the IS
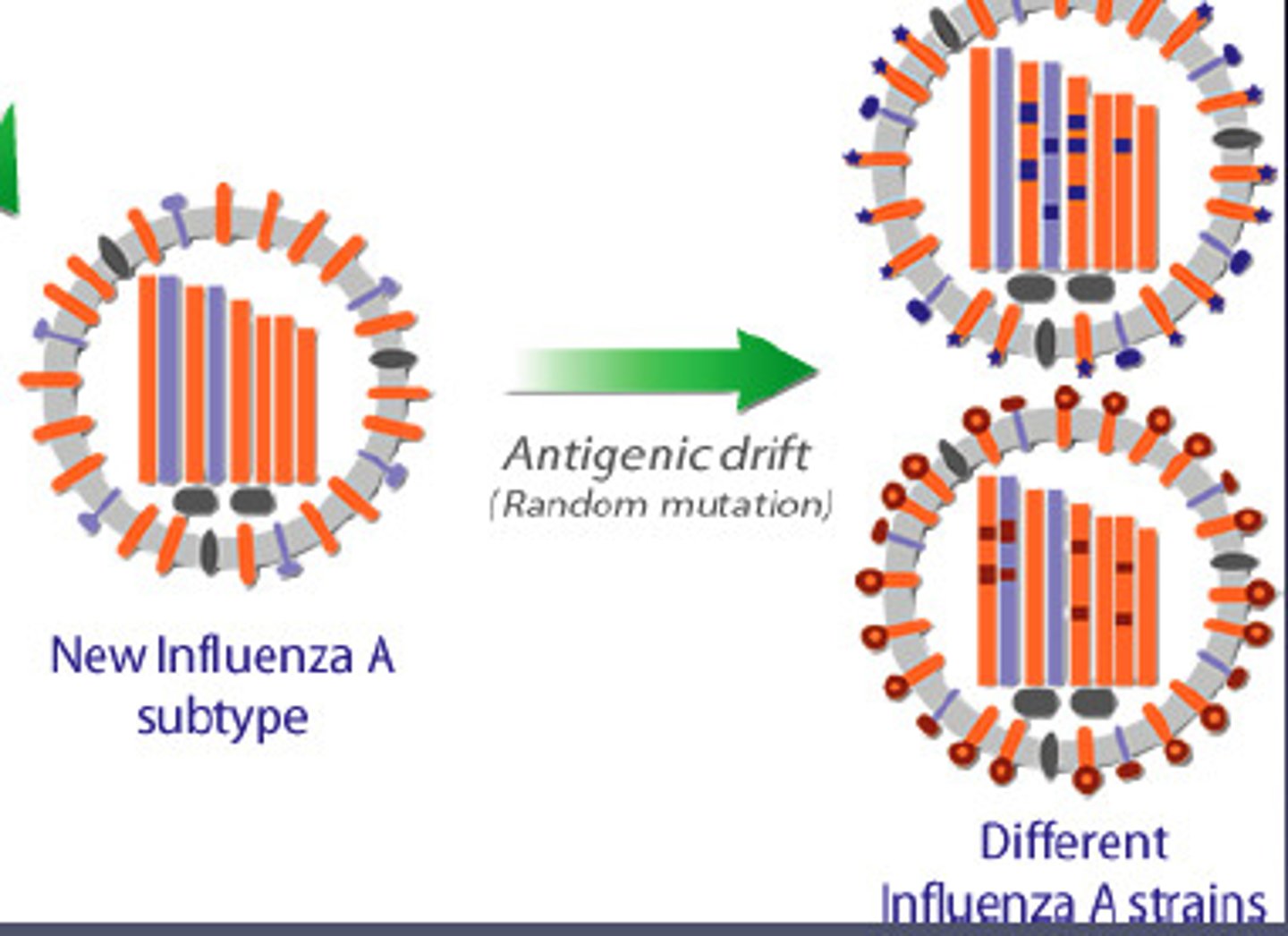
Strategy to beat IS - antigenic drift
Changes in antigens due to mutations in pathogen
DNA profiling - step 1
(obtaining)
Obtain DNA sample:
- cheek cells obtained by mouth swabbing
- WBC obtained in blood sample
DNA profiling - step 2.1
(enzymes)
Create DNA fragments
- use restriction enzymes, they cut DNA at specific recognition points
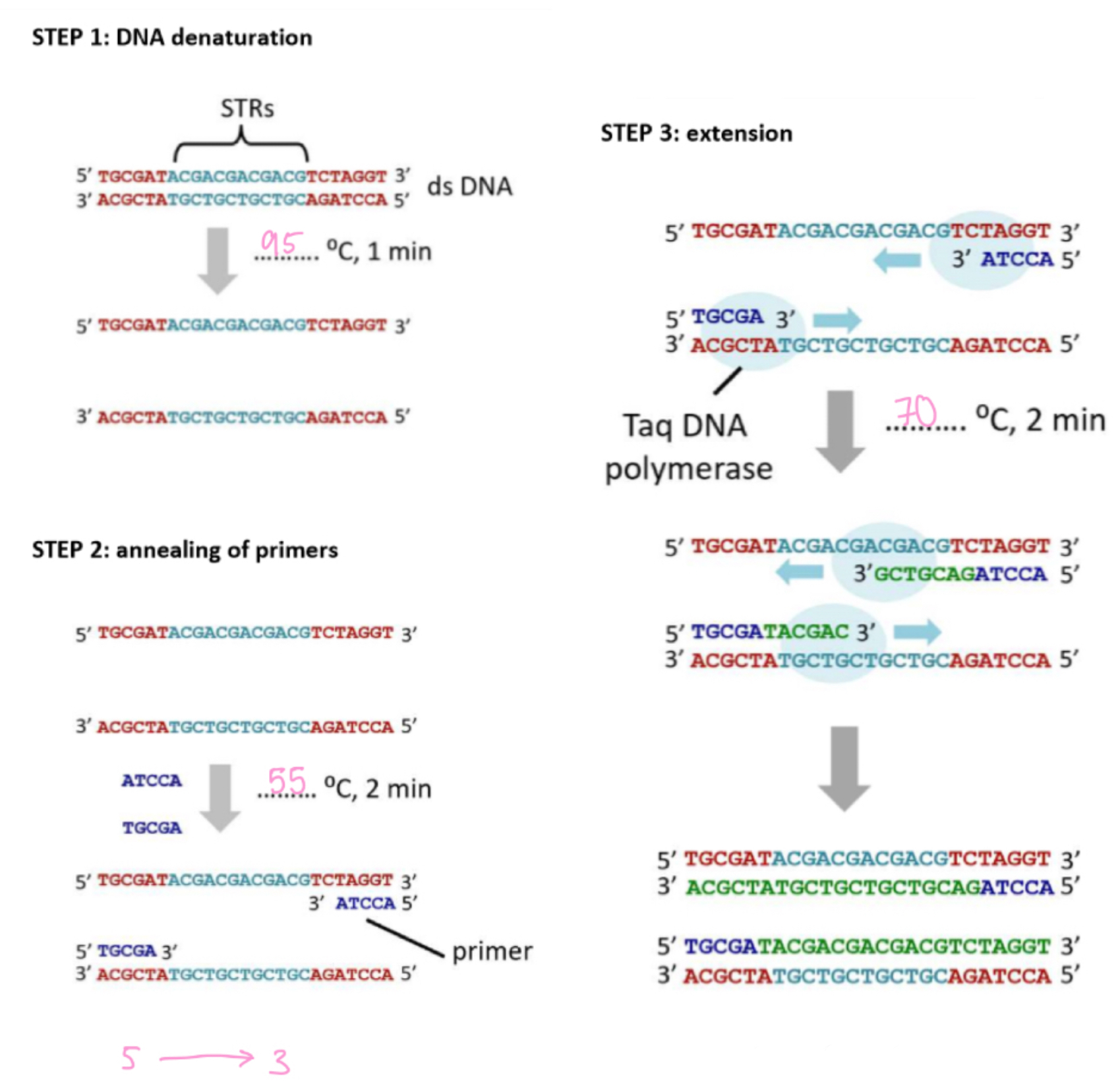
DNA profiling - step 2.2
(the remaining 4 steps)
Create DNA fragments
step 1: DNA denature
- DNA strands are separated, hydrogen bonds broken / 95°C
step 2: annealing of primers
- small primers attack at the start & end of STP sequence via complementary base pairing / 55°C
step 3: extension
- Taq DNA polymerase synthesises complementary DNA strands (5' → 3') using free nucleotides / 70°C
step 4: amplification
- steps 1 to 3 are repeated 25-30 cycles, in every cycle more DNA is present to act as a template
Gel electrophoresis
A method of separating DNA fragments according to size in an agarose gel by applying an electric field
Visualising the DNA banding pattern
DNA can be stained using ethidium bromide then visualised under a UV lamp
- it glows in UV light making DNA visible in gels
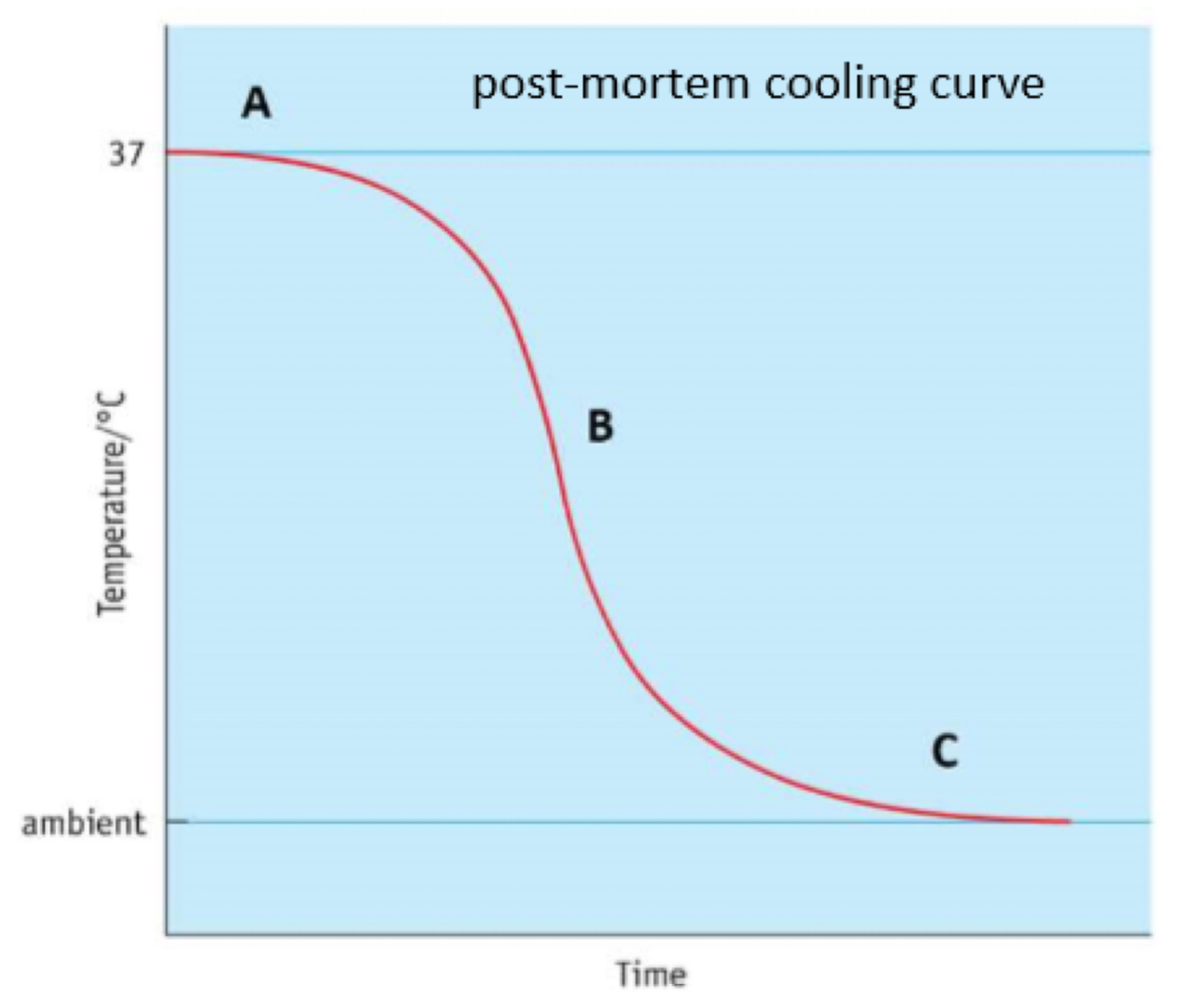
Body temperature & death graph explained
A:
- sigmoid curve
- plateau, 30-60 min
- metabolic reactions not fully stopped yet
B:
- linear decline of temperature can be
used to estimate time of death
(~ 1.5⁰C /h)
C:
- body temperature reaches ambient
(environmental) temperature
What affects initial core body temperature
Hypothermia
Fever
Rigor Mortis
After death muscles first relax, then stiffen, and then relax again
Decomposition
Digestion of cells resulting in breakdown of tissues and release of carbon and nutrients (e.g. nitrate and phosphate)
1. Fresh
(initial decay)
0 to 3 days
- autolysis
- anaerobic bacteria in gut start to digest tissues & release gases (start of putrefaction)
2. Bloating
(putrefaction)
3 to 10 days
- increasing gas production by bacterial activity causes swelling of body & putrid odour
- breakdown of haemoglobin leads to venous marbling of skin + green discoloration of abdomen
3. Active decay
(black putrefaction)
10 to 20 days
- discolouration of skin (changes to purple then black)
- tissue start to soften and then liquefy
- flesh looks creamy
- loss of fluid and deflation of body
4. Advanced decay
20 to 50 days
- majority of internal tissue lost
- body starts to dry out
Succession
Insects arrive on a corpse in a predictable sequence depending on the stage of decomposition
Forensic Entomology
Study of insects and other small invertebrates
in criminal investigations
What does a forensic entomologist do
- take samples of larvae
- take temperature of environment
- keep maggots to determine species
- kill maggots to determine age to determine time of death
Succession in entomology
1. Bacteria will be found in and on the dead body immediately after TOD
2. As tissue decomposition sets in it creates ideal conditions for flies to lay eggs and their larvae to hatch
3. As more soft tissue is consumed by the fly larvae it creates favourable conditions for beetles to establish
4. When tissue dries out over time flies will leave the body as they prefer a moisture-rich environment
5. Beetles, however, can decompose dry tissue so they will remain on the body
6. Once all tissues have been decomposed most organisms will leave the body
What factors affect the rate of decomposition
Weather
Exposure (e.g. curled up = less exposure = less decay)
Temperature (e.g. hot/humid = encourage bacteria growth = rate of decomp increases // cold = slows down rate oof decomp)
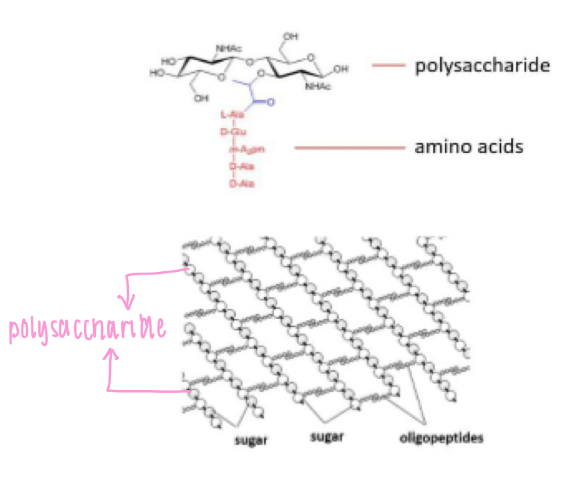
Bacterial cell wall components
- Peptidoglycan (polysaccharides held together by
oligopeptide cross-links)
- Oligopeptide
(small peptide 2-20 aa)
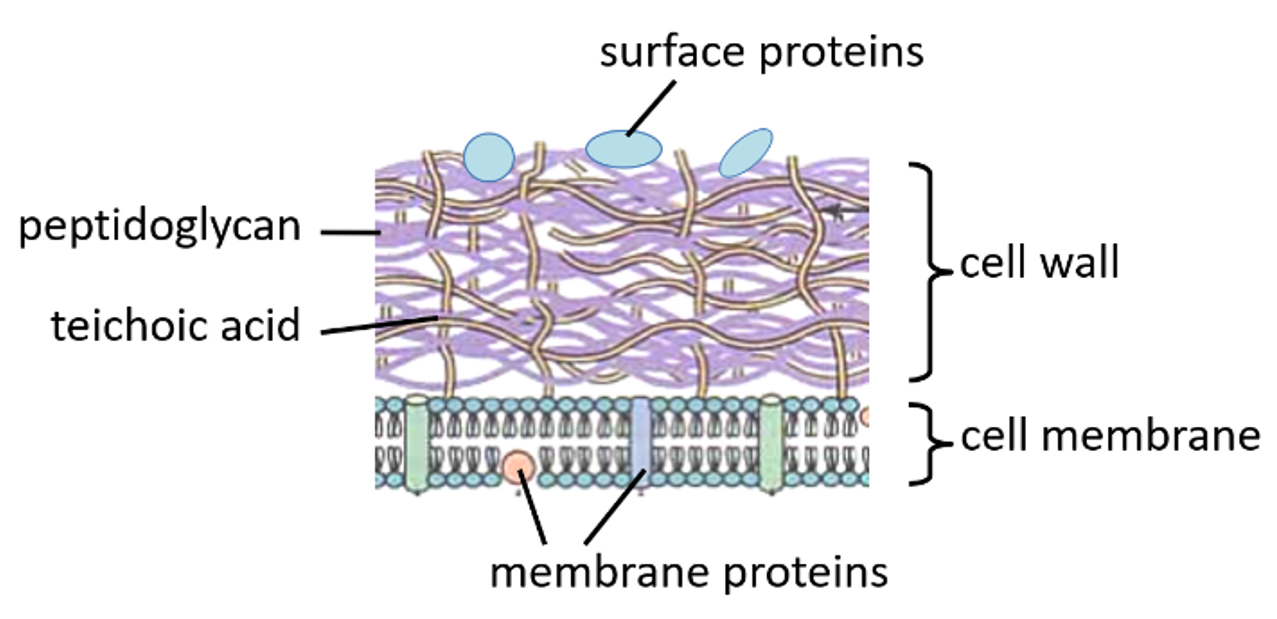
Gram-positive bacterial cell wall
Thick layer of peptidoglycan
One cell membrane
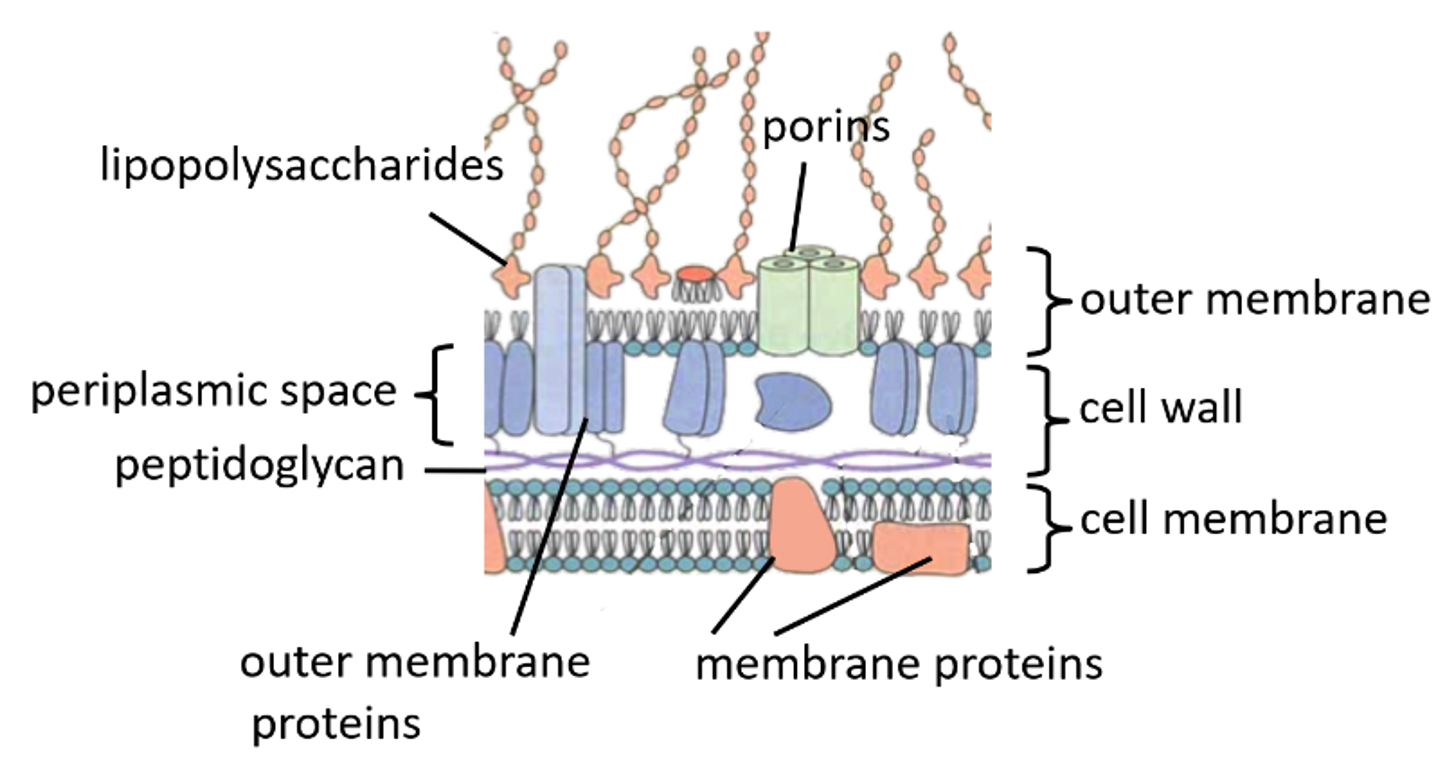
Gram-negative bacterial cell wall
Thin layer of peptidoglycan
Cell membrane and another outer cell membrane
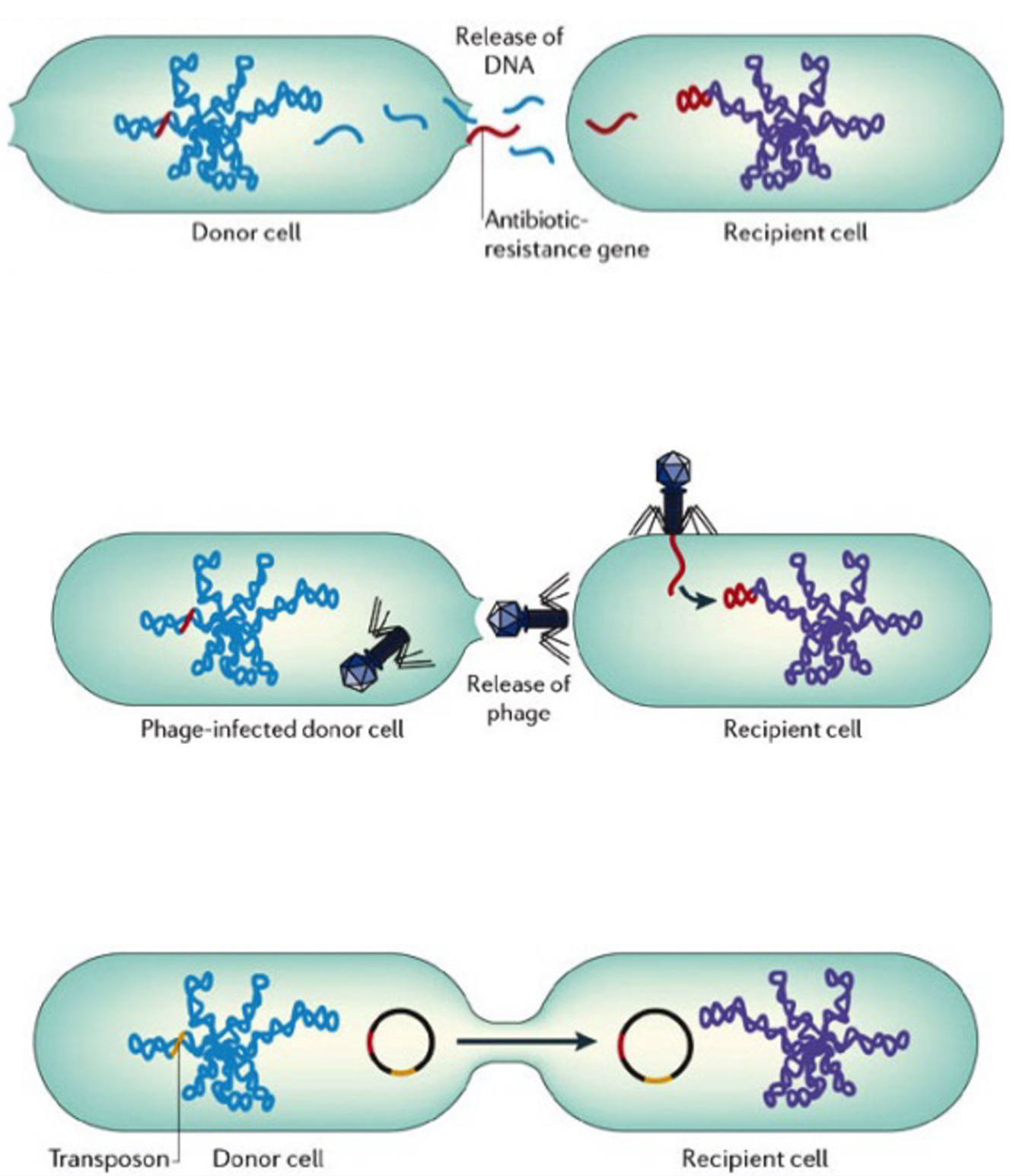
Bacterial reproduction
Asexual reproduction by binary fission via horizontal gene transfer
1. Transformation: DNA is taken up from environment & may be integrated into bacterial DNA
2. Transduction (via bacteriophage): bacterial DNA transferred to other bacteria by bacteriophages
3. Conjugation (via pilus): DNA passed through cytoplasm in pilus to another bacterial cell
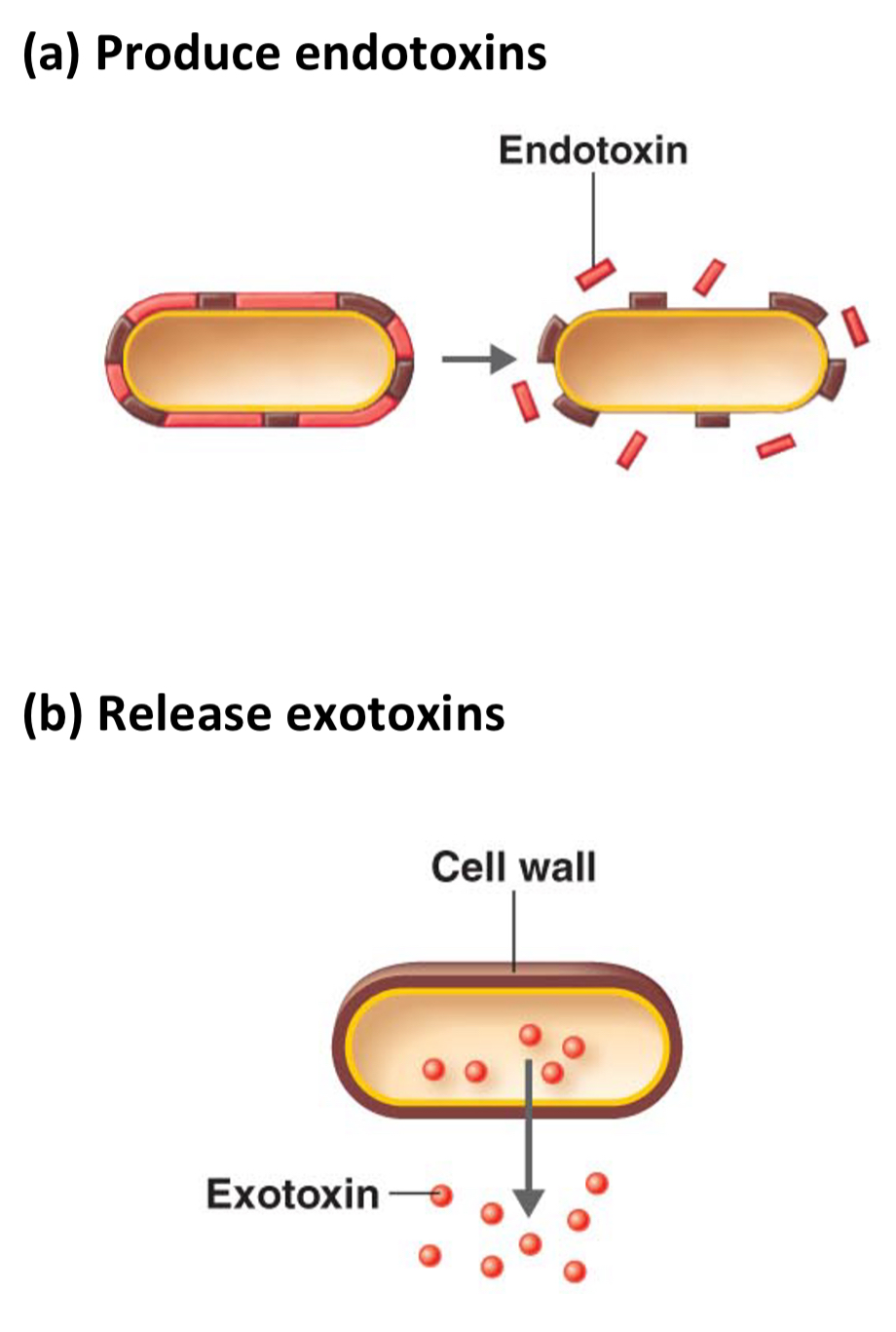
How bacteria cause illness
1. Produce endotoxins - cause vomiting, diarrhoea, fever
2. Release exotoxins - toxic effect on cells, inhibit neurotransmitters etc
How do viruses cause illness
1. destruction of host cell during lysis
2. hijack host cell's protein synthesis so slow down host cell's metabolism
3. produce toxins
Immune system structure
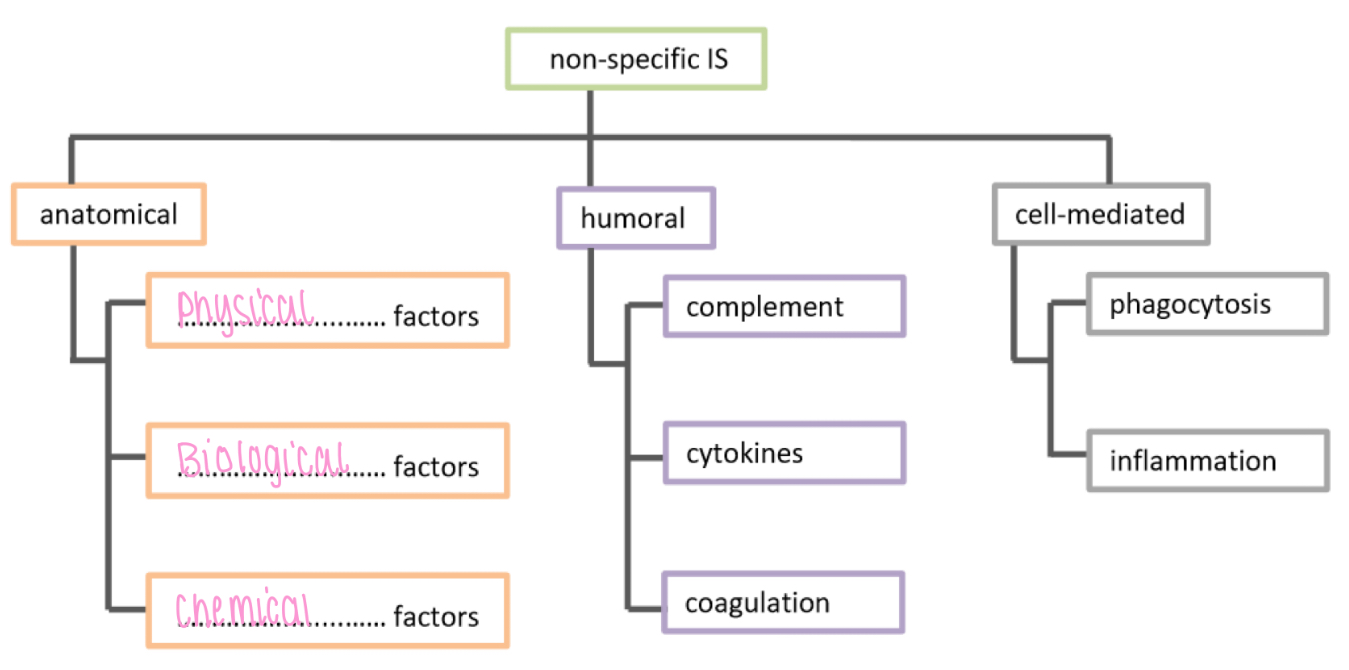
Non specific IS - anatomical, physical factors
Airways/epithelium: ciliated cells
Skin: keratin, makes skin waterproof
Non specific IS - anatomical, chemical factors
Ears: earwax
Mouth: saliva, mucus
Nose + eyes: tears, lysozyme
Skin: sebum
Stomach: stomach acid
Vagina: acidic secretion
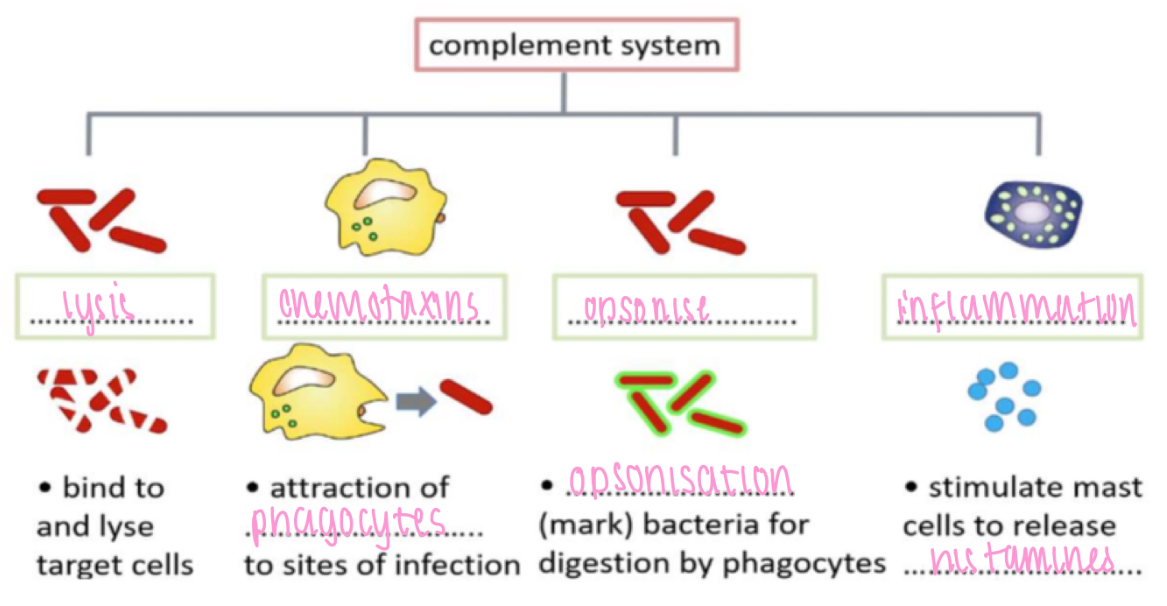
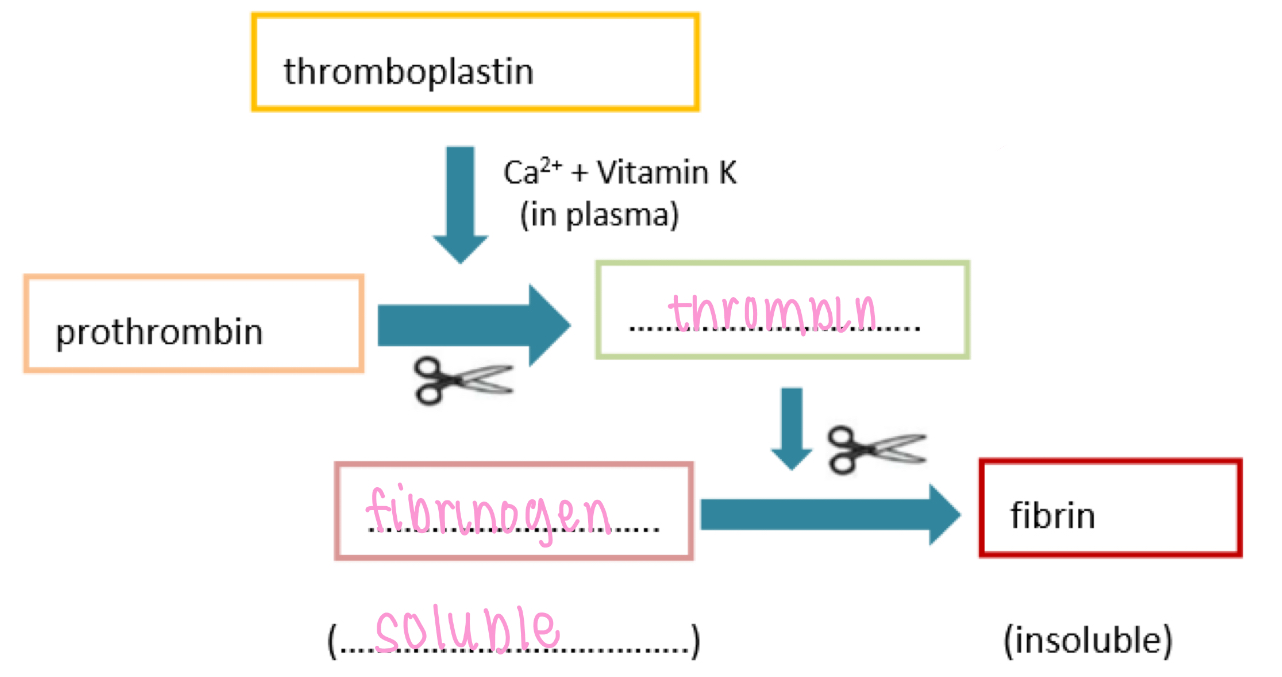
Non specific IS - humoral, complement
Lysis - bind to & lyse target cells
Chemotaxis - attraction of phagocytes to sites of infection
Opsonise - opsonisation (mark) bacteria for digestion by phagocytes
Inflammation - stimulate mast cells to release histamines
Non specific IS - humoral, cytokines
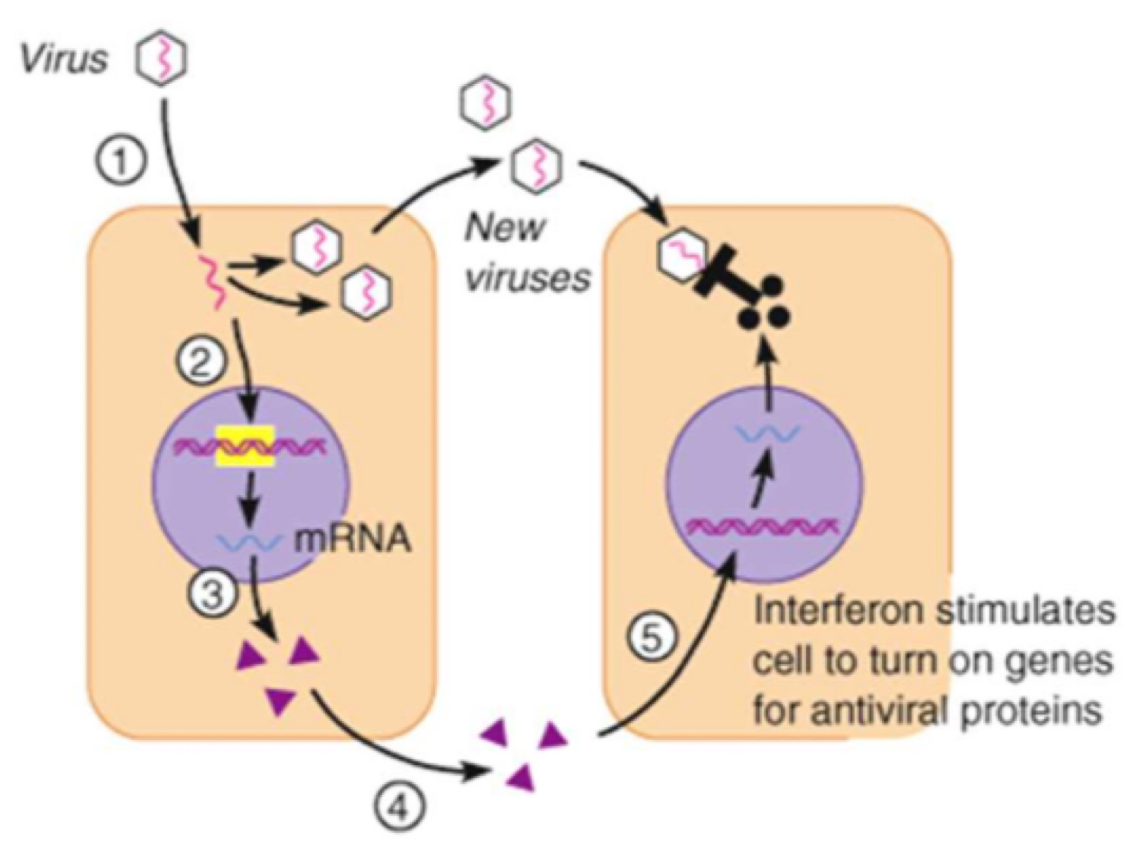
Example: interferons
Produced by virus-infected cells
Induces virus resistance in uninfected cells
They inhibit the production of viral proteins, preventing the virus from replicating
They activate white blood cells involved with the specific immune response to destroy infected cells
They increase the non-specific immune response e.g. by promoting inflammation
Non specific IS - humoral, coagulation
Phagocytosis
Digest and engulf pathogens
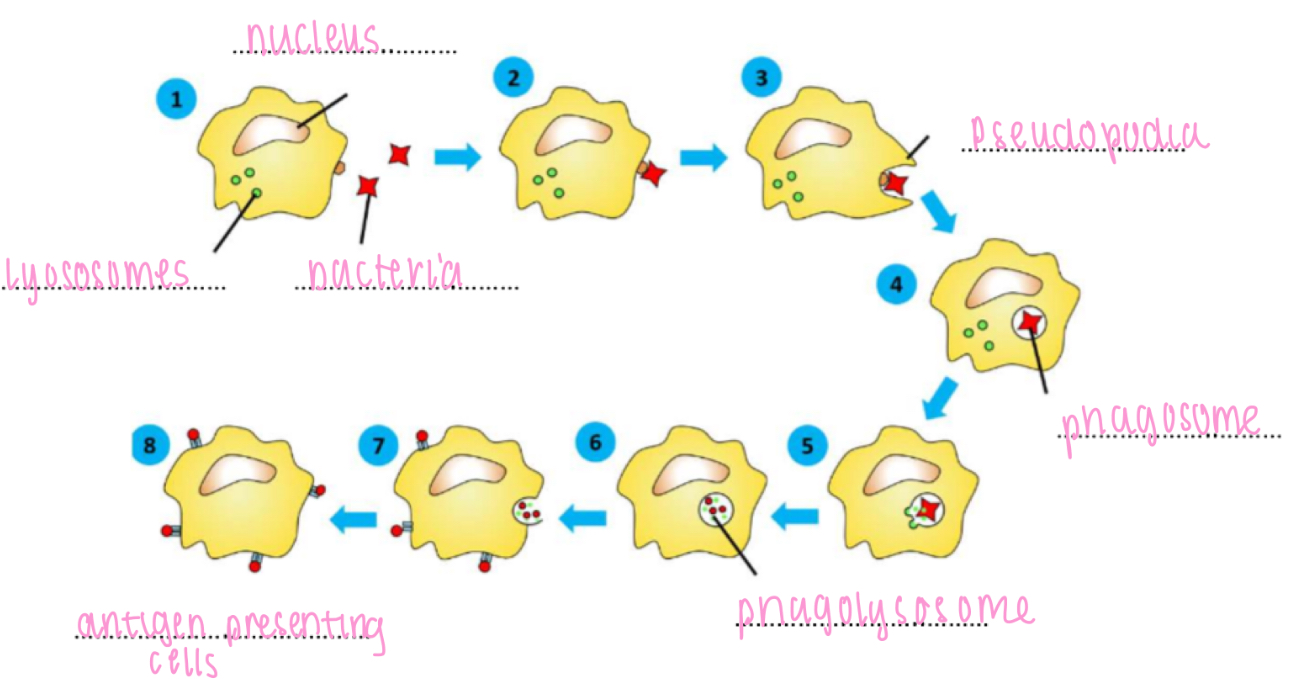
Non specific IS - cell-mediated, phagocytosis
1. movement towards bacteria by chemotaxis
2. bind to bacterium
3. engulf bacterium
4. phagosome formation
5. phagosome and lysosomes fuse
6. digestion of bacterium by lysosome enzymes / destruction with superoxide
7. egestion of bacterial debris
8. antigen presentation (monocytes and macrophages only)
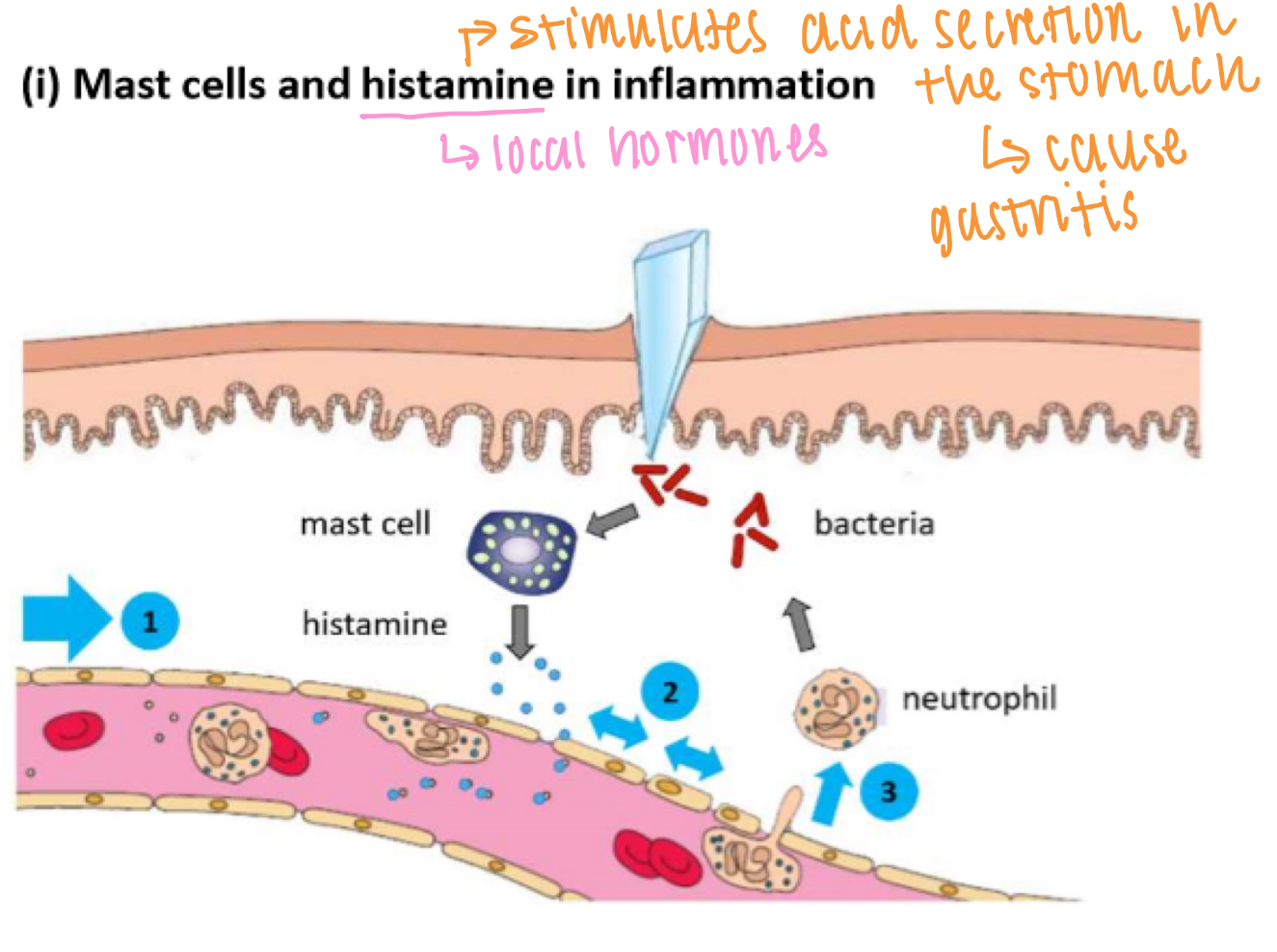
Non specific IS - cell-mediated, inflammation
1. Mast cells release histamines
2. Causes vasodilation → increased blood supply (redness + heat)
3. Causes increased vascular permeability (endothelial cells contract) → swelling & pain
4. More WBC arrive to clear bacteria
Specific IS - humoral, B cells
Mature in bone marrow
Differentiate into memory B cells & plasma B cells (produce antibodies)
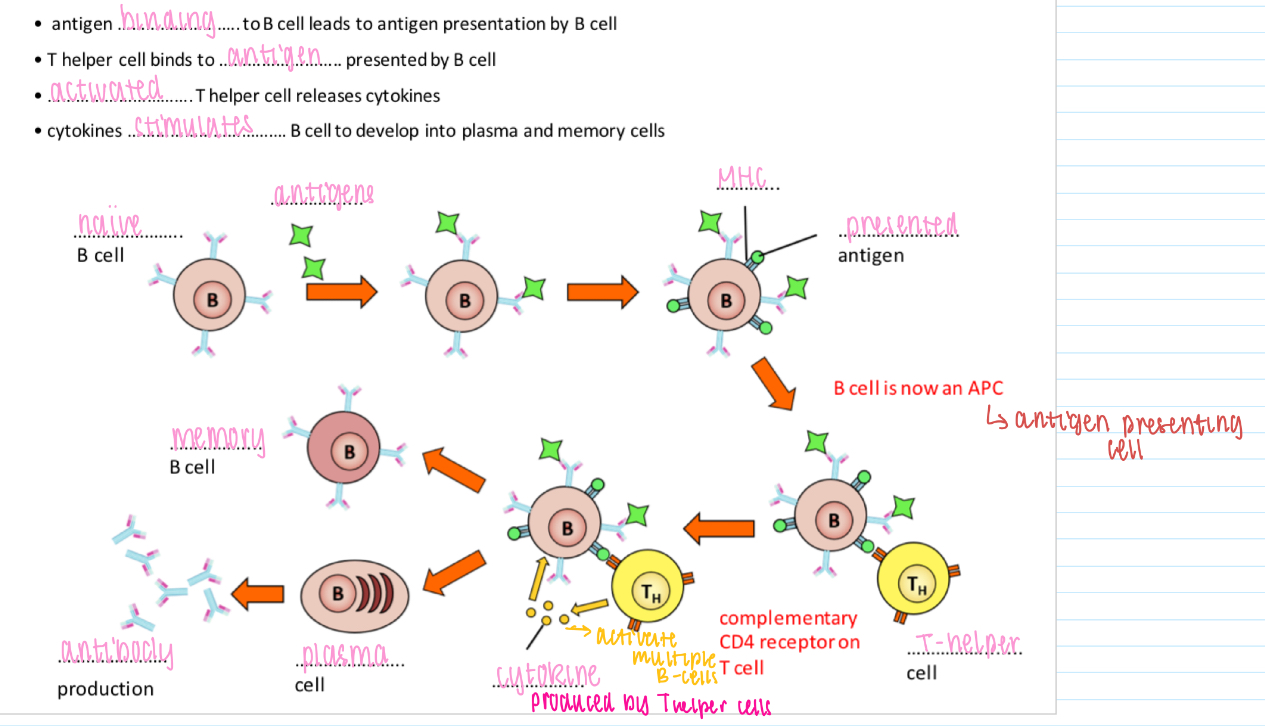
B cell activation - T cell-dependent
Antigen binding to B cell leads to antigen presentation by B cell
T helper cell binds to antigen presented by B cell
Activated T helper cell releases cytokines
Cytokines stimulates B cell to develop into plasma and memory cells
PICTURE ON WS
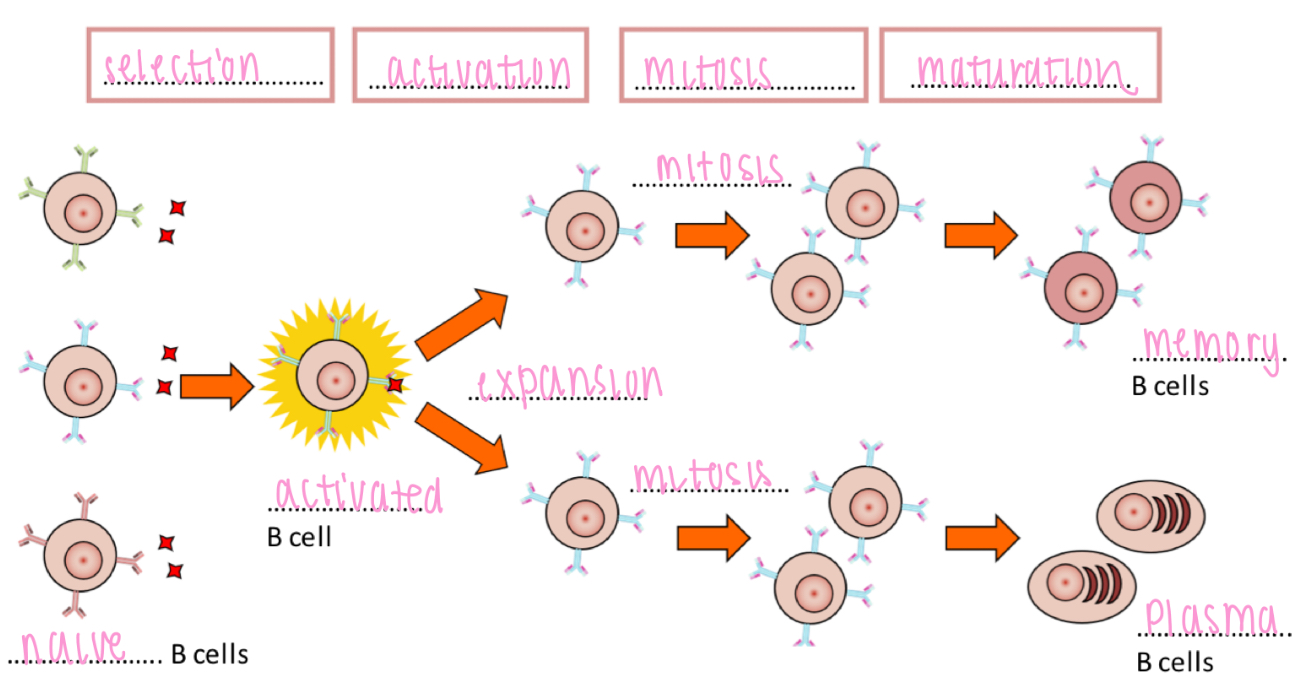
Clonal expansion - B cell activation
Selective B cell activation leads to clonal expansion and maturation
- Selection → Activation → Mitosis → Maturation
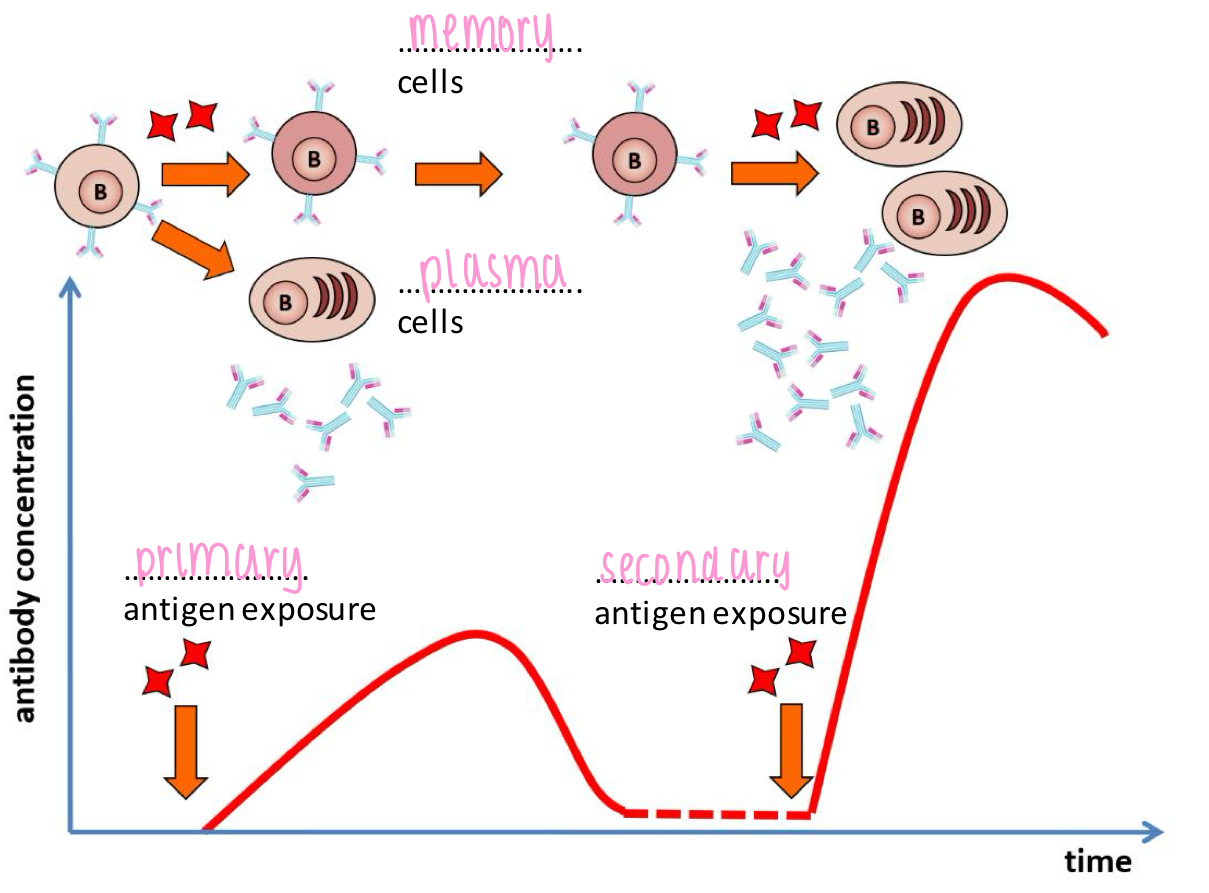
Primary and secondary immune response
Primary immune response:
- time lag
- short-lasting immunity
- fewer antibodies
Secondary immune response:
- immediate
- long-lasting (booster)
- more antibodies
Role of antibodies
1. Opsonisation - pathogens phagocytes more readily
2. Compliment activation - cell lysis
3. Agglutination - antibodies bind to pathogens causing them all to stick together
4. Neutralisation
Specific IS - cell-mediated, T cells
Mature in thymus
Differentiates into:
1. T-helper cells
2. T-killer cells
3. T-memory cells
4. T-suppressor cells
T cell receptor
Recognises processed antigen bound to MHC
Antigen binding to TCR trigger activation of T cell
Co-receptor (T helper cell co-receptor = CD4)
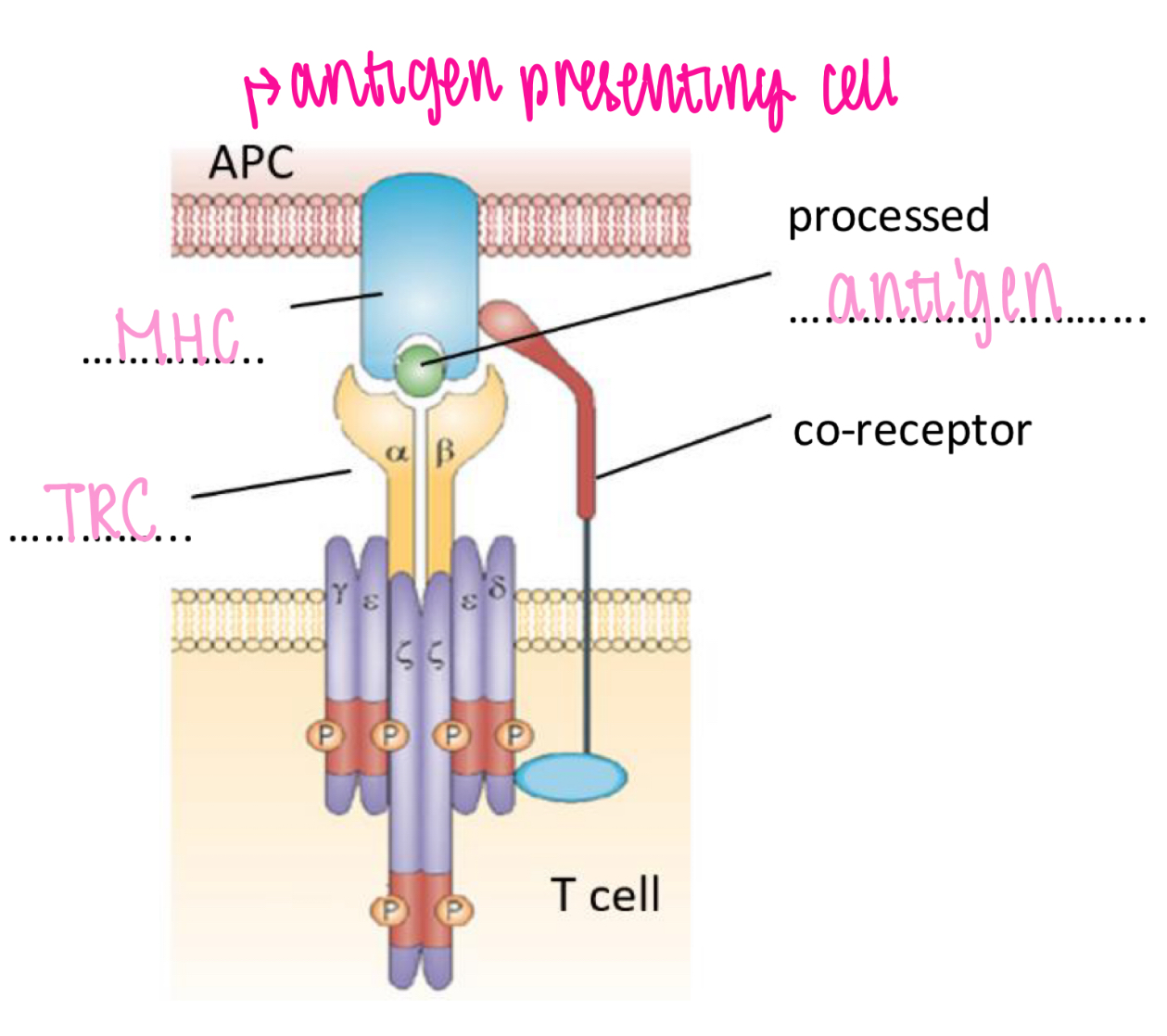
T cell activation
PICTURE ON WS
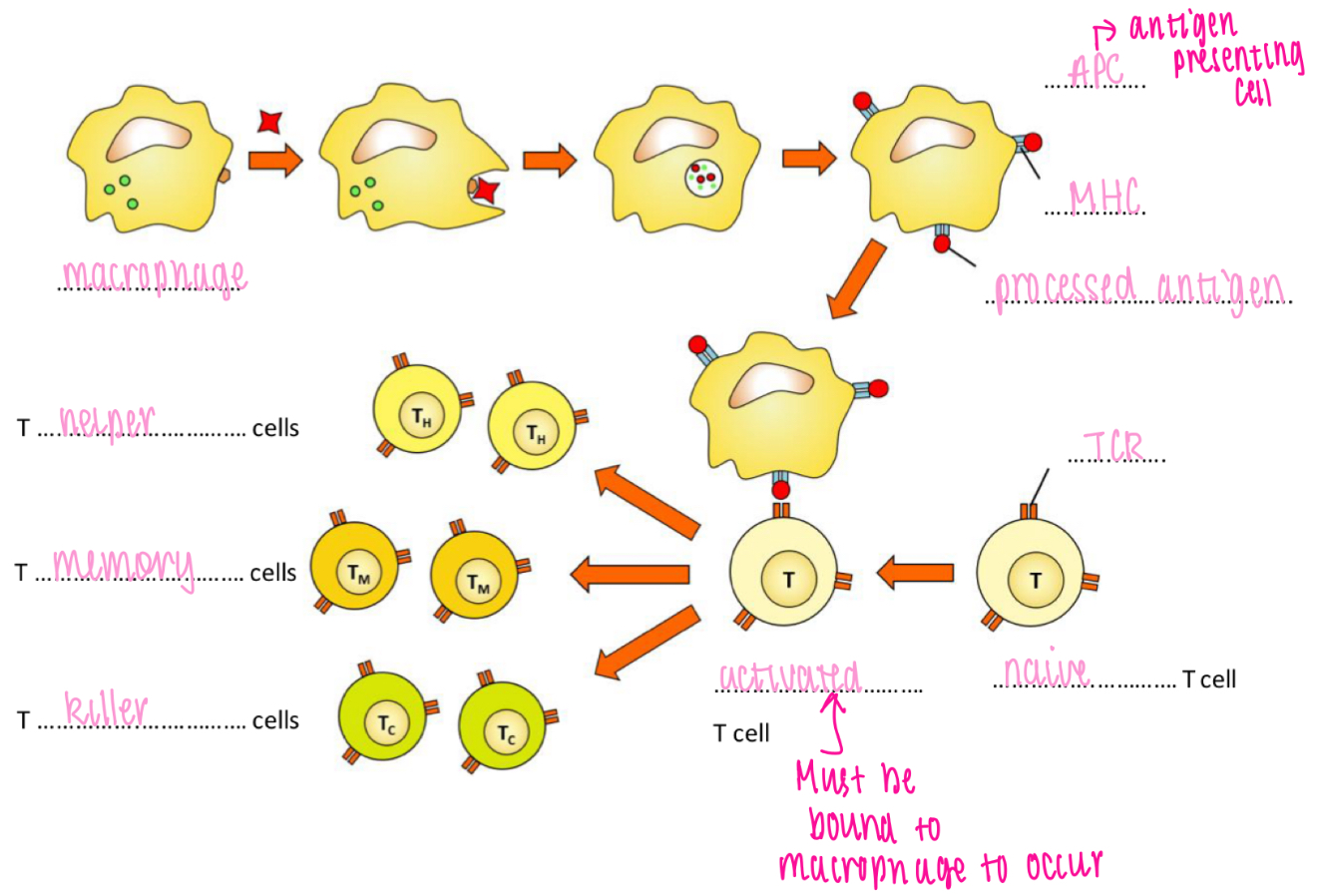
Clonal expansion - T cell activation
T cell activation leads to differentiation and clonal expansion
- Selection → Activation → Mitosis → Maturation
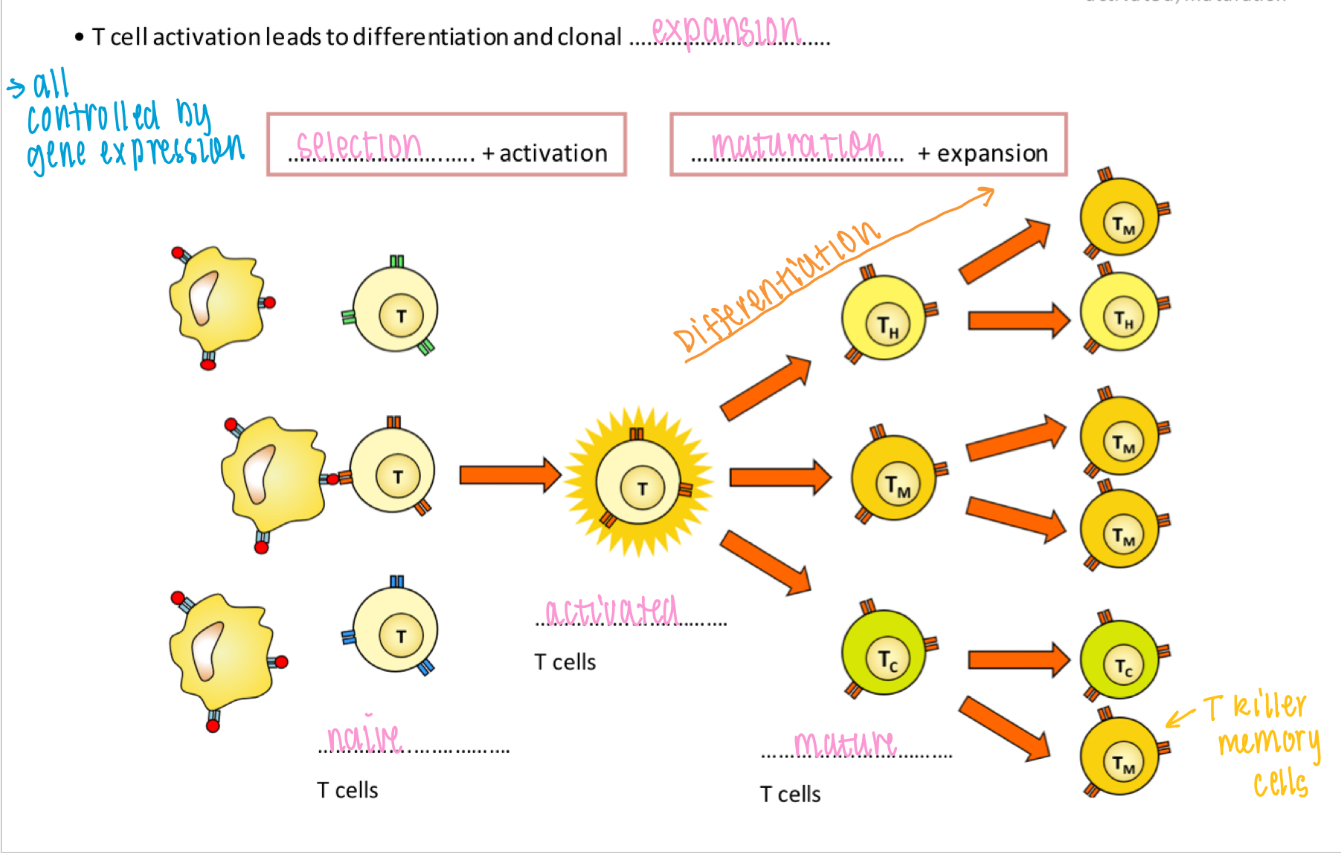
Role of T helper cells
1. B cell activation
2. Phagocyte enhancement (by opsonisation of microbes)
3. T killer cell activation
Mycobacterium tuberculosis - first phases
1. Macrophages ingest bacteria, can't digest them
2. TB can reproduce or become dormant inside
macrophage
3. Group of infected/uninfected macro, B/T cells form a granuloma
4. Granuloma becomes encapsulated forming a tubercle
- inside TB is starved of O₂ SO TB & macrophages die
- infection often fought off + lung tissue heals
Mycobacterium tuberculosis - evading the IS
Taken up by phagocytosis cannot be destroyed due to their thick waxy capsule
TB bacteria can survive inside macrophages & lie dormant for years
Reactivation occur at a later point
Mycobacterium tuberculosis - secondary phases
(when it occurs, organs effected, symptoms)
Secondary phase occurs when...
1. too many bacteria
2. immune system is weakened
Lungs: rapid multiplication & destruction of lung tissue
Disease could spread to: glands, bones, CNS
Symptoms:
- coughing (up blood)
- shortness of breathe
- loss of appetite/weight loss
- fever & night sweat
- fatigue
Mycobacterium tuberculosis - diagnosis
1. Skin test
- injection of antigen
- if antibodies present, inflammation occurs
2. Blood tests
- test for presence of TB antigen specific T cells
3. Analysis of bacteria
- sputum coughed up is tested for bacteria species
- cell wall stain
- DNA analysis
4. Chest X-ray
Mycobacterium tuberculosis - treatment & control
1. Antibiotics
- streptomycin
2. Improved living standards to control transmission
3. Screening by X-ray
4. Immunisation (BCG vaccine)
Fever - process + how it aids the IS
1. Bacteria consumed my macrophage
2. Macrophage releases interleukin 1 (IL-1)
3. IL-1 causes hypothalamus to release prostaglandin
4. Prosta resets hypothalamic thermostat
to new higher BT (inhibit/denature bacterial enzymes)
5. Shivering, higher metabolic rate, inhibition of sweating & vasodilation
- increases phagocytosis, no. T cells & decreases reproduction of pathogens
AIDS
Acquired immune deficiency syndrome
- increases susceptibility to other diseases (opportunistic infections, cancer, etc...)
HIV - transmission
Unprotected sex with infected partner
Sharing needles with infected person
Transmission from infected mother to foetus (breastfeed or placenta)
Infection from blood transfusions
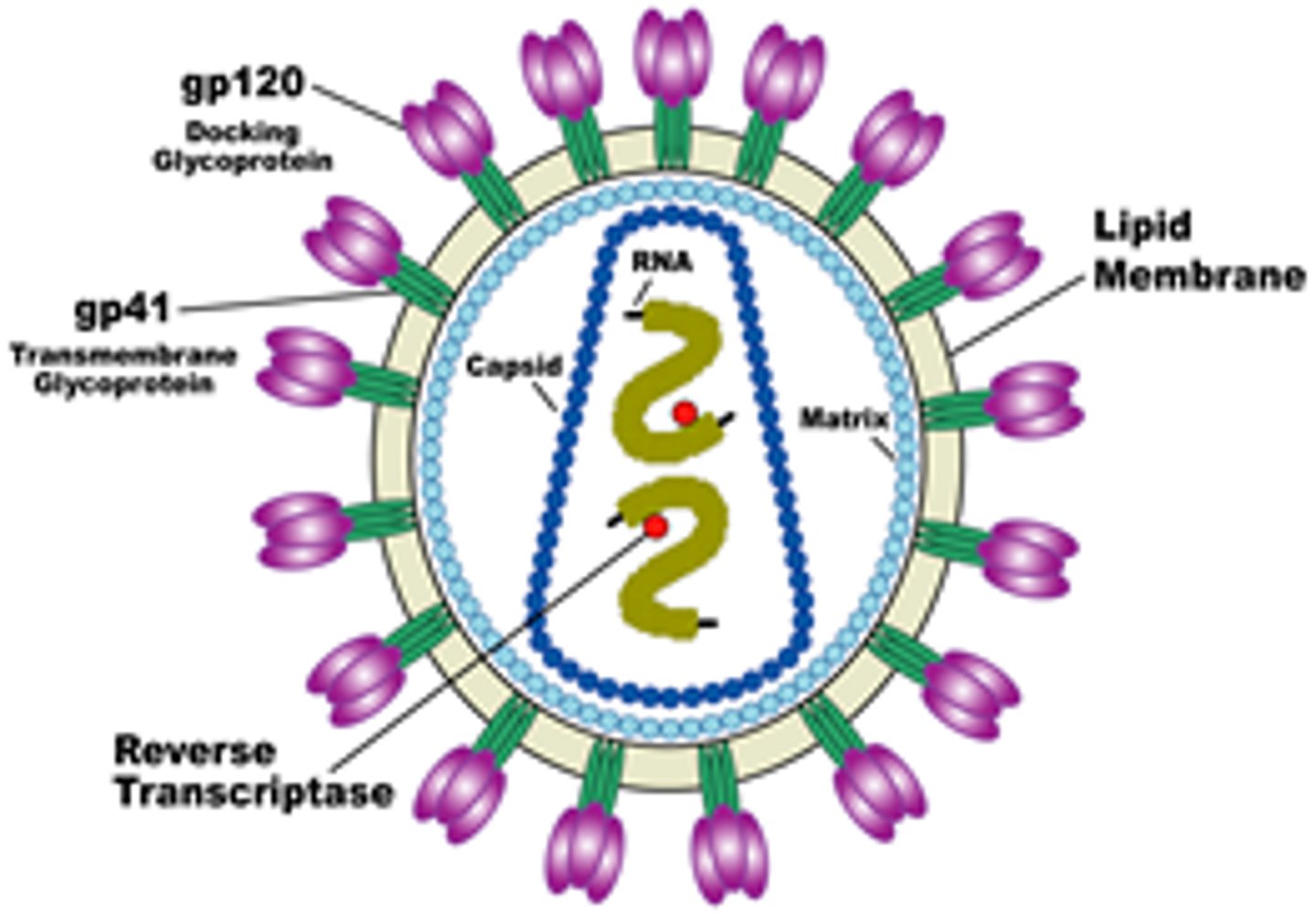
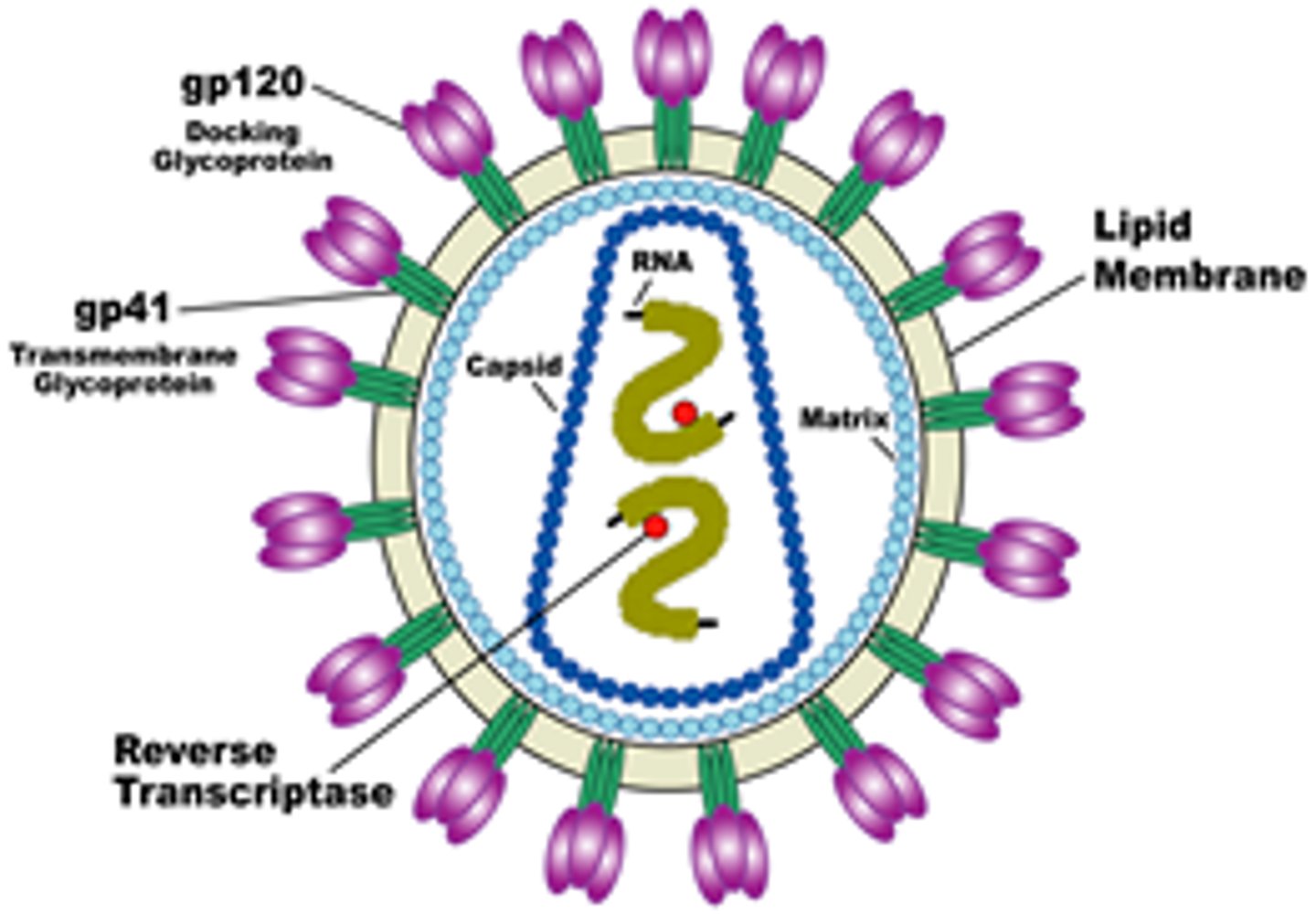
HIV - structure of virus
Retro virus, spherical
- 2 copies of ssRNA w/ 9 genes
- gp120 docking glycoprotein
- gp41 transmembrane glycoprotein
- capsid protein
- lipid membrane (envelope)
- viral enzymes (reverse transcriptase, integrase, protease
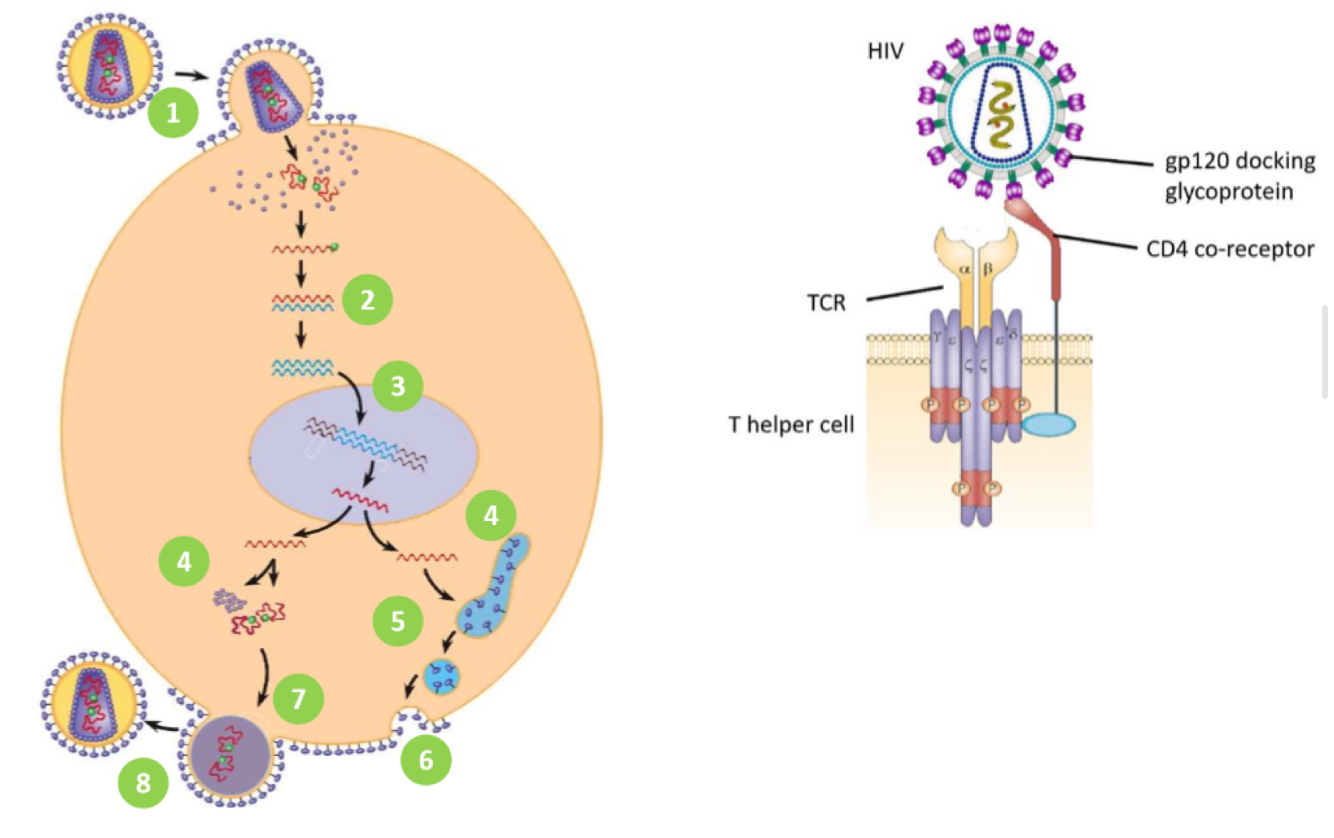
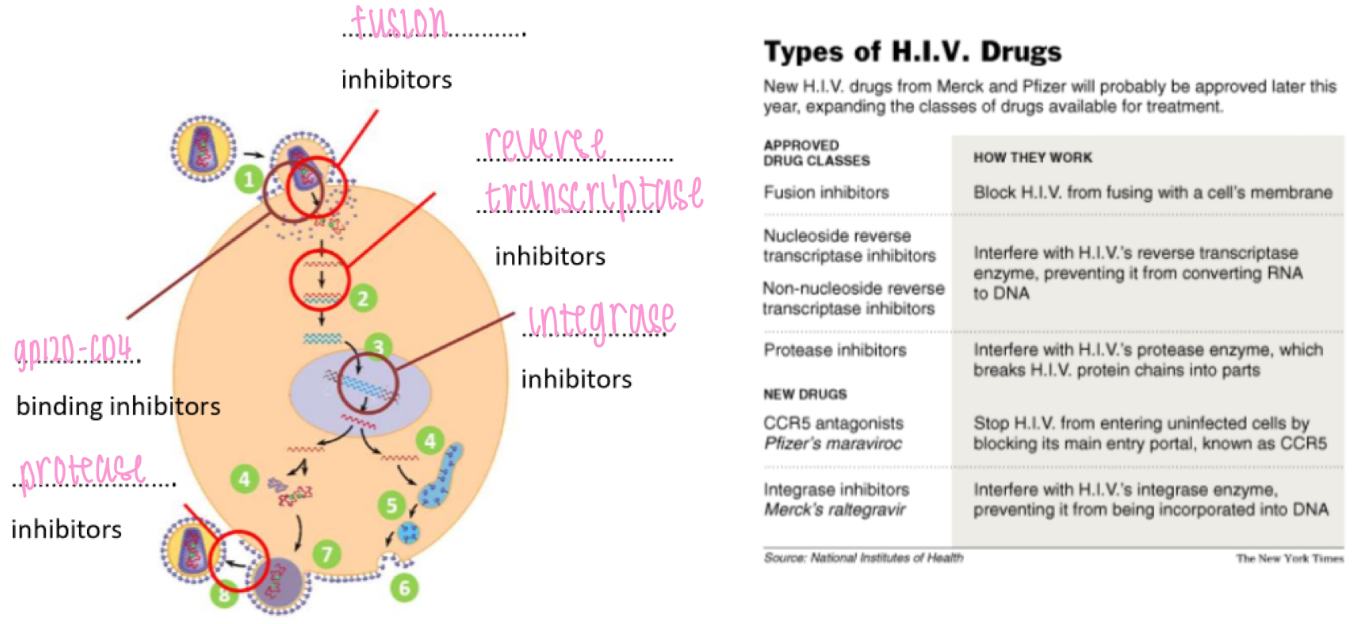
HIV - life cycle
1. HIV gp120 docking protein binds to CD4 co-receptor on Tₕ cells
• HIV fuses w/ cell membrane & releases ssRNA into host cell
2: reverse transcriptase copies viral ssRNA to make dsDNA
3: viral DNA is transported into nucleus
• integrase inserts viral DNA into host DNA
4: transcription and translation of viral envelope protein gp160
• transcription of viral DNA into RNA to be used as new virus genome
• translation of RNA to make new viral polyprotein
: viral envelope protein gp160 passes through secretory pathway where it is cleaved to form
gp120 + gp41
6: viral envelope proteins are incorporated into host cell membrane
7: new viral RNA and polyprotein move to cell surface and new immature HIV virion forms by
budding from cell membrane
8: virus matures when HIV protease cleaves individual HIV proteins and enzymes from polyprotein
• virus is now infectious
HIV - acute phase
2-6 weeks post infection:
- possible influenza like symptoms
- rapid replication of virus
- rapid loss of Tₕ cells
3-12 weeks post infection:
- antibodies appear in blood
8-12 weeks post infection:
- infected Tₕ cells recognised and destroyed by Tₖ cells
HIV - disease phase aka AIDS
Rapid decline of Tₕ cells
Rapid increase in viral load
HIV-related symptoms appear:
- weight loss, fatigue,
diarrhoea, night sweats, etc.
- later stages: major weight loss, dementia, cancers
Risk of opportunistic infections:
- TB, pneumonia, etc.
- death
HIV - drugs (ARVs)
Fusion inhibitors: block HIV from fusing w/ a cell's membrane
(Non)/-nucleoside reverse transcriptase inhibitors: interfere w/ reverse transcriptase enzyme, preventing conversion of RNA to DNA
Protease inhibitors: interfere w/ protease enzyme, breaks HIV's protein chains into parts
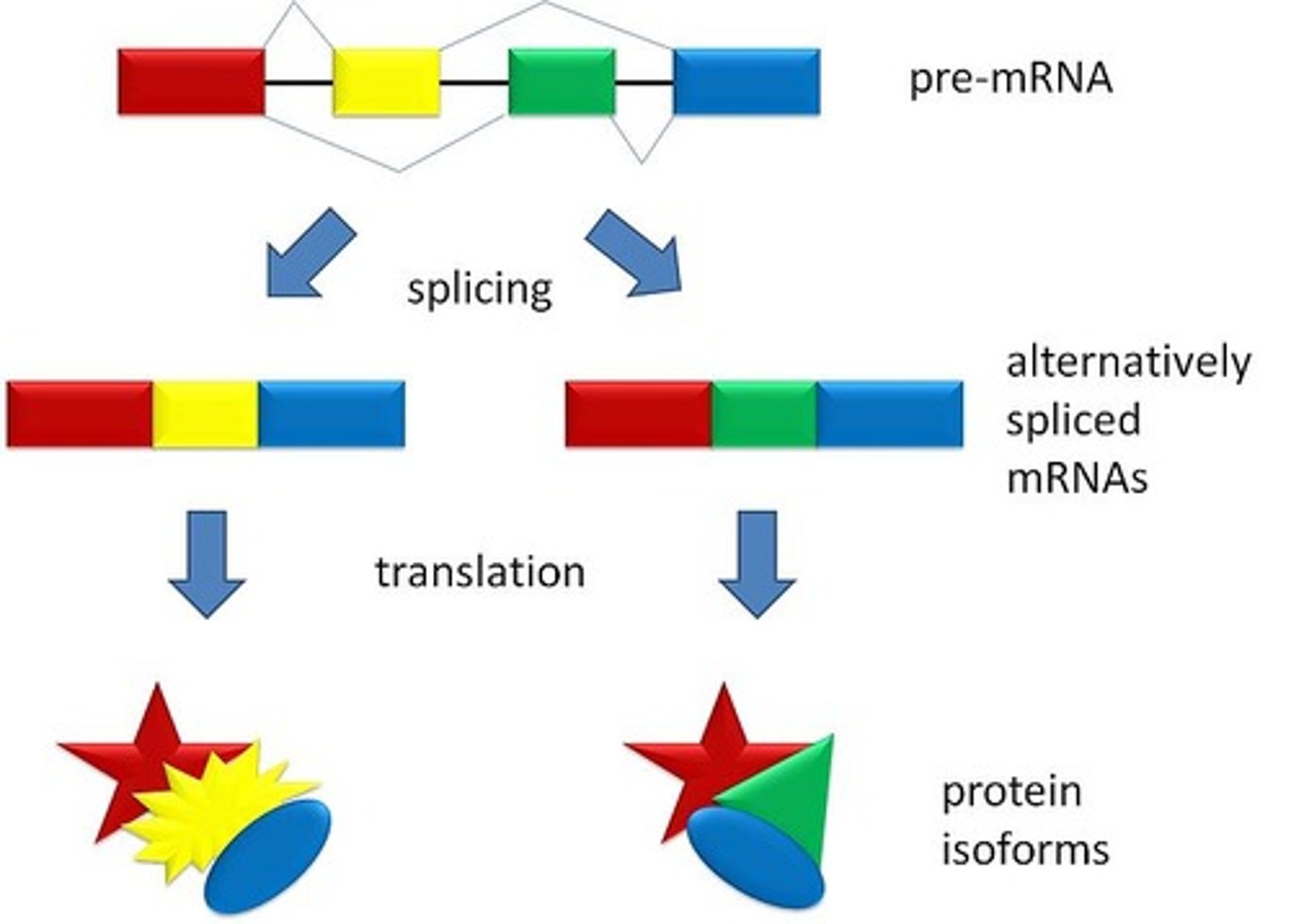
Post-transcription modification of RNA
1. Splicing: removal of introns
2. Alternative splicing: allows different proteins to be produced when different exons are spliced together
Immunity
Having sufficient B & T memory cells to avoid disease
Active immunity + types
Acquired when an antigen enters the body triggering a specific immune response
- produces memory cells → provides long-term immunity
1. Natural: exposure to pathogens
2. Artificial: vaccination
Passive immunity + types
Acquired without an immune response; antibodies are gained from another source, not produced by the infected person
- no memory cells
1. Natural: foetus via placenta or through breast milk
2. Artificial: injection/transfusion of antibodies or antibodies collected from animals/people who's IS has been triggered by vaccination to produce antibodies
Booster injection
Provides re-exposure to antigens
- increase no. T & B memory cells to maintain protective levels
Vaccines - advantages
-Fewer people get ill and die
-Herd immunity may be achieved - wider
population protection (even those who are not
immunised)
-R₀ (reproduction number) kept low
-Harmful outbreaks of disease are prevented,
e.g. of influenza in vulnerable populations
(over 65s)
-Fewer people hospitalised so burden on healthcare services reduced
Vaccine - disadvantages
-No vaccine gives 100%. protection and vaccine
efficacy varies from one type of vaccine to the next
-Risk of adverse reactions, e.g. inflammation at the site of injection, fewer,
or allergic reactions
Bacteriostatic:
Prevent multiplication (host's IS can then destroy pathogen)
- target protein synthesis
- target DNA replication
- target RNA transcription
Heterozygous advantage
Where a selection pressure for the heterozygous genotype keeps a genetic disease allele in a population
Strategy to beat IS - TB
- resistant to digestion by lysosome's enzymes
- survive inside macrophage
- destroy macro + infect others
Strategy to beat IS - HIV
- antigenic drift & shift
- HIV infects Tₕ cells
- lies dormant
- kills Tₕ cells
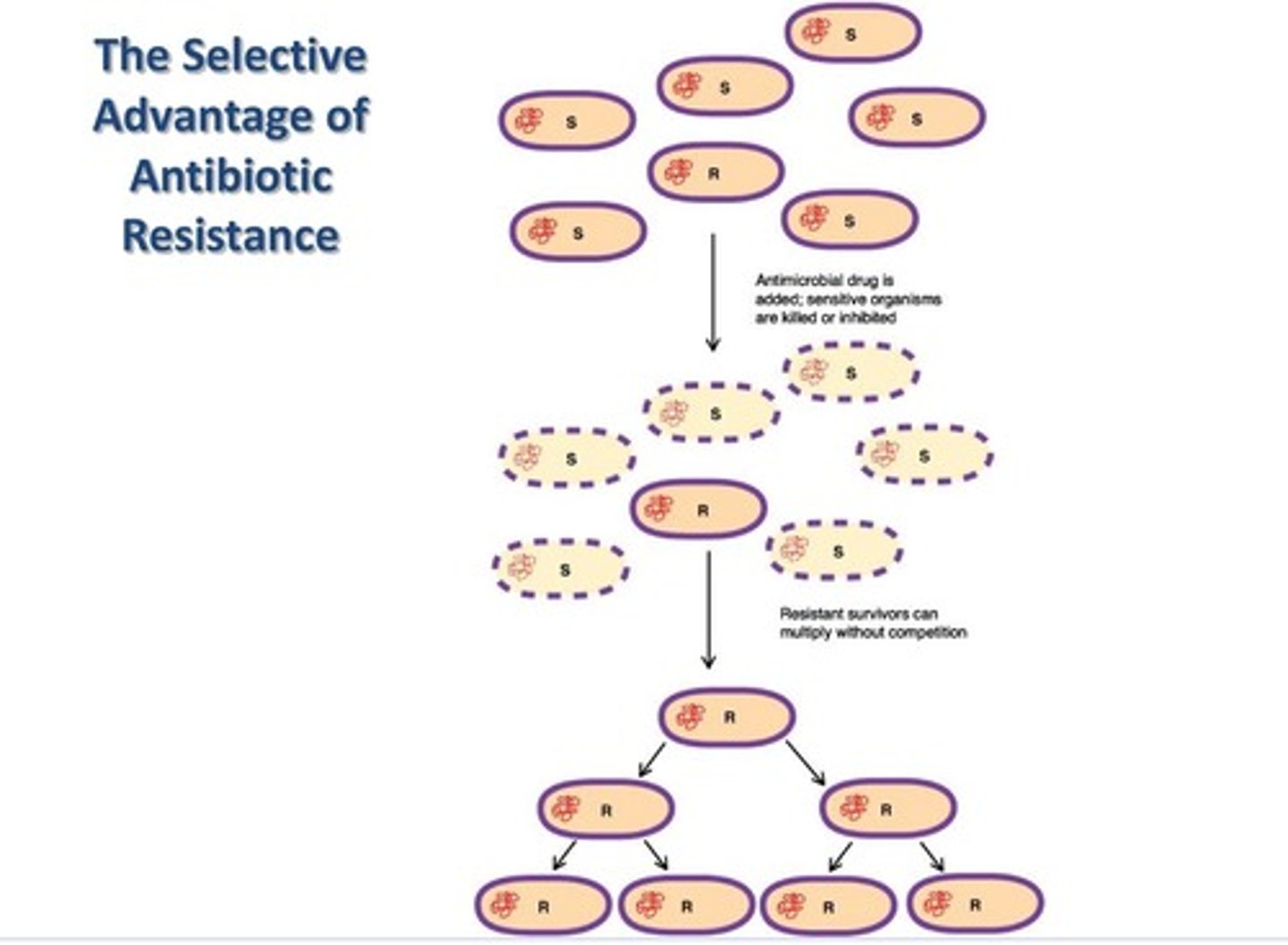
Strategy to beat IS - antibiotic resistance
- due to mutations
- due to horizontal gene transfer
Strategy to beat IS - natural selection
just basic natural selection process
Preventing HealthCare Associated Infections (HCAIs)
1. Better hygiene
- more hand wash stations
- signs reminding people to wash hands
- changes in clothing (no ties, no long sleeves)
- thorough cleaning of wards)
2. Fewer antibiotics used
- only used when bacterial infection confirmed
3. Isolation of infected patients