chem 2- multiple bonds (*exam 1)
0.0(0)
Card Sorting
1/3
Earn XP
Description and Tags
Advanced theories of covalent bonding
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
what happens when a double bond occurs, how is the geometry of that?
a pi bond from a double or a single bond results from the overlap of the remaining 2p orbital on an atom that is not involved in hybridization
it will be perpendicular to the plane of the sp² hybrid orbitals
the unhybridized orbitals overlap in a side by side fashion above and below the internuclear axis forming a pi bond.
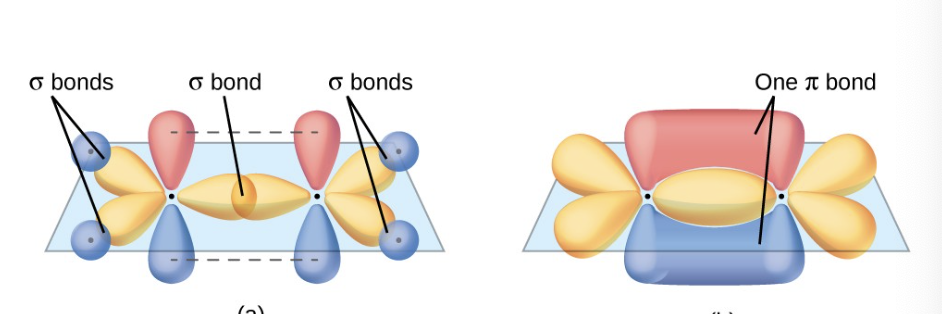
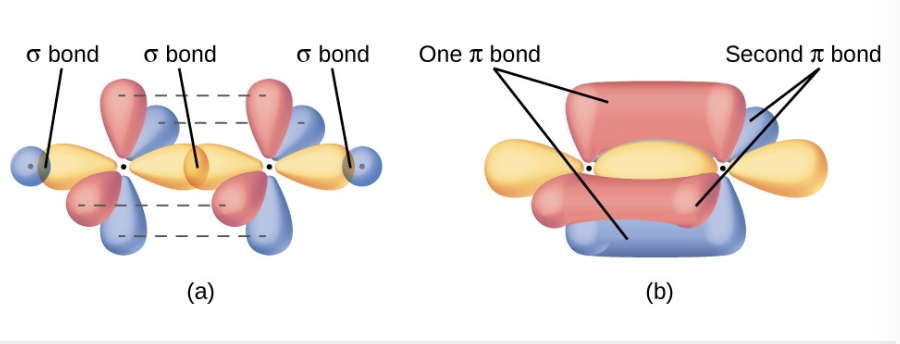
2
New cards
when do resonance forms occur
when various arrangements of pi bonds are possible.
does NOT influence the assignment of hybridization
the electrons are not located in one position or the other, but the are actually delocalized throughout the ring.
3
New cards
well ig this was a short unit
4
New cards
srrrrrryyyy ;(