MD 4. Carboxylic Acid Derivatives: Nucleophilic Substitution Reactions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
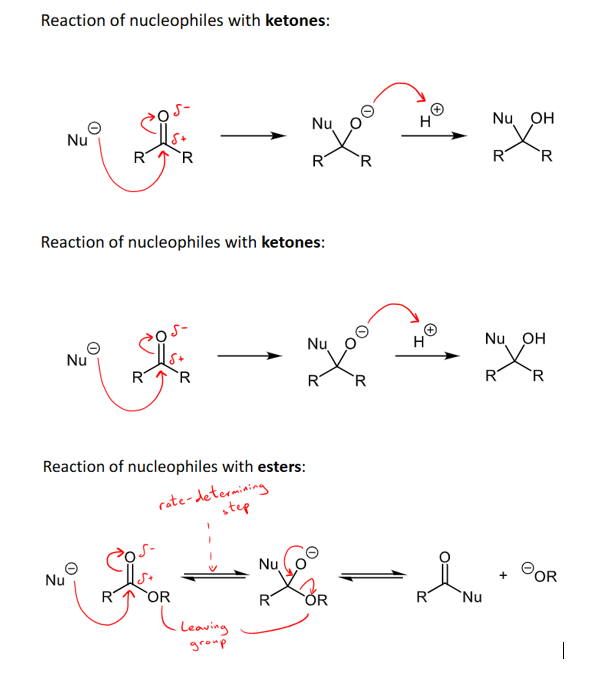
What are the differences in the way ketones, carboxylic acids and esters react with nucleophiles?
ketones react with nucleophiles under nucleophilic addition reactions
Esters react with nucleophiles under a nucleophilic substitution reaction - the OR group is removed and replaced with the nucleophile, leaving the carbonyl on
Carboxylic acids react under competitive deprotonation to form RCOO- and NuH - although nucleophilic acyl substitution can occur sometimes
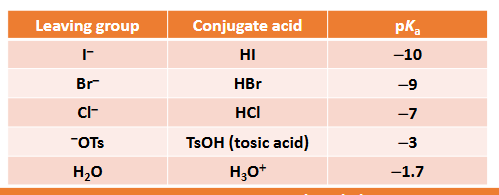
What are leaving groups? What are the ‘best’ leaving groups?
leaving group accept a lone pair of electrons as the bond between it and its neighbour is broken
best leaving groups are neutral molecules, water, and stable ions, Cl-
How does pKa link to leaving group ability?
the more stable the ion is, the better it will be as a leaving group and the larger the equilibrium constant will be - the equilibrium constant is the acid dissociation constant, Ka
pKa = -log10 (Ka)
Therefore, the more stable A- is, the higher the Ka and the smaller the pKa so a lower pKa value for the conjugate acid, HA, makes A- a better leaving group
What is the relative reactivity for aldehydes, ketones, esters?
esters react slower than ketones and aldehydes as the Or group is a +M mesomeric group making the C less delta positive
And aldehydes react the quickest still due to only one +I effect and less steric hinderance
And ketones are in the middle with two +I effects causing the C to be slightly less delta positive and there to be more steric hinderance with two R groups
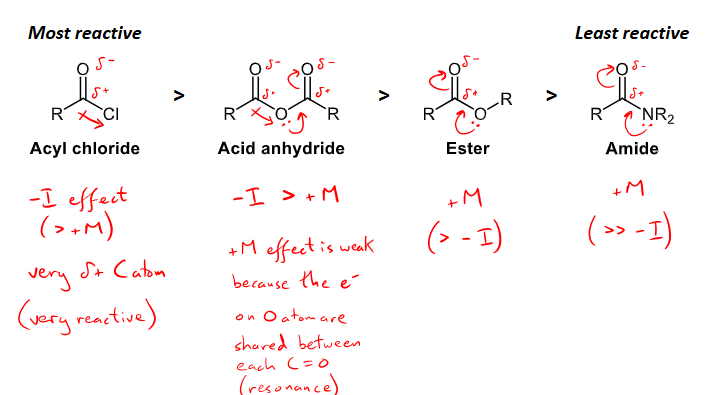
What is the relative reactivity of carboxylic derivatives
fast nucleophilic acyl substitution needs a strong nucleophile and electrophilic carboxylic derivatives.
based on the electrophilicity of the carbon, the most to least reactive is: acyl chloride, acid anhydride, ester, and lastly amide is the least reactive.
acyl chlorides have a very strong -I effect from the Cl, making the carbon more positive and more reactive
acid anhydrides have a weak +m effect and a much stronger -I effect as there is electron resonance from the central O atom making a more positive C
esters have a +m effect making the carbon a less positive
Amides have a strong +M effect making the carbon less positive.
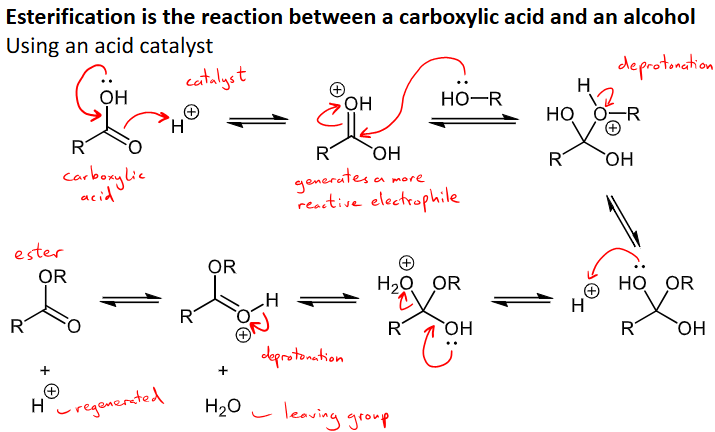
Outline the reaction and mechanism for the esterification of a carboxylic acid
carboxylic acid + alcohol = ester + water
uses an acid catalyst
All steps are reversible however so there are two ways to push the equilibrium towards ester formation: 1) use excess alcohol and 2) remove water from reaction mixture as it is formed
acid H+ catalyst reacts with carboxylic to make a reactive electrophile that reacts with the alcohol, then the ROH group is deprotonated, the other OH group reacts with H+ to form H2O+ on the molecule, lone pair from the other OH is moved to this H2O, which is our leaving group
the proton attached to the O on the newly formed C=O bond is then removed and this is the regeneration of our catalyst
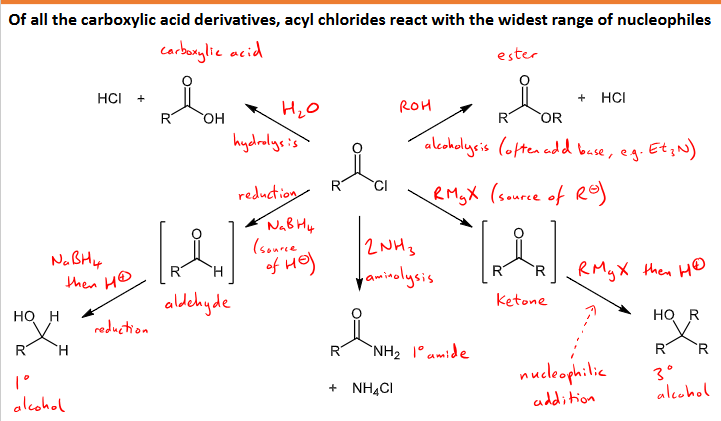
What reactions (5) with what conditions can acyl chlorides undergo?
they can undergo the widest nucleophilic reactions as the most reactive
hydrolysis - reaction with water to form carboxylic acid and HCl
alcoholysis - reaction with alcohol to form an ester and HCl
Grignard reagent substitution and then addition - makes ketone intermediate through substitution of the reagent R group replacing the Cl and then RMgX and H+ is added to undergo addition and form a tertiary alcohol
aminolysis - reaction with 2NH3 to form a primary amide and NH4Cl
Reduction - NaBH4 reducing agent, forms aldehyde intermediate, then reacted with NaBH4 and H+ to reduce to a primary alcohol
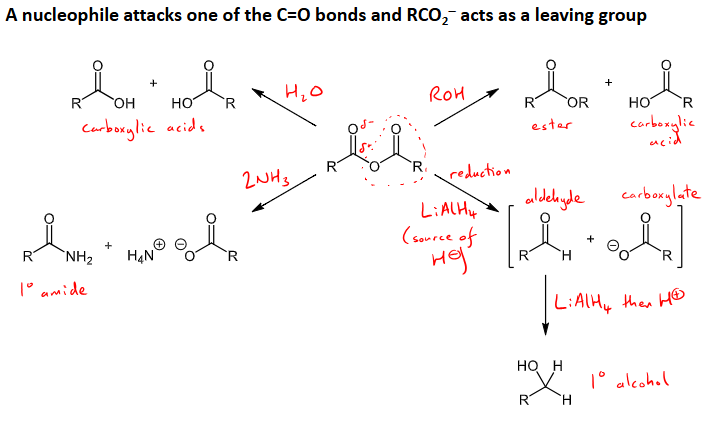
What reactions (4) with what conditions can acid anhydrides undergo?
nucleophile attacks the C=O and the RCO2 acts as a leaving group
hydrolysis - addition of water - forms two carboxylic acid molecules
alcoholysis - addition of alcohol to form an ester and a carboxylic acid
reduction - LiAlH4 reducing agent to source H-, forms an aldehyde and carboxylate ion intermediates, then add LiAlH4 and H+ to form a primary alcohol.
Aminolysis - Addition of two ammonia molecules (2NH3) to form a primary amide and an ionic H4N+OCOR molecule
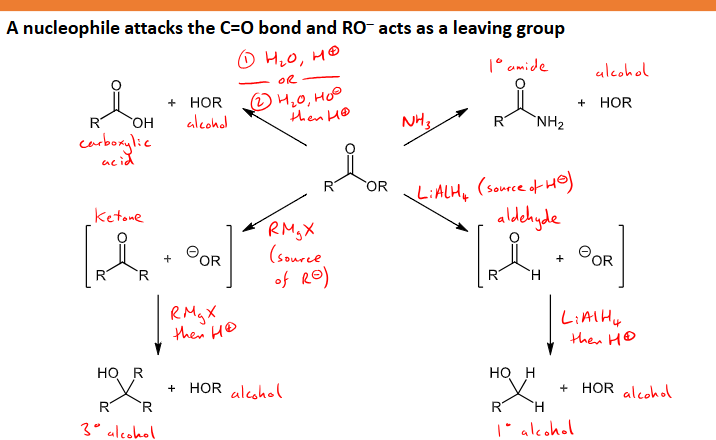
What reactions (4) with what conditions can esters undergo?
Nucleophile attacks C=O and RO- acts as leaving group
reaction with ammonia (NH3) to form a primary amide and an alcohol
Reaction with LiAlH4 to form aldehyde and OR- intermediates, react with more LiAlH4 and H+ to form a primary alcohol and another alcohol
Grignard Reagent - Reacts with RMgX to substitute the R group in and make a ketone and OR- intermediate, react with another RMgX molecule and H+ to form a tertiary alcohol and another alcohol
hydrolysis/de-esterification - React with water and H+ (or base hydrolysis with water, OH- and then H+) to form an alcohol and carboxylic
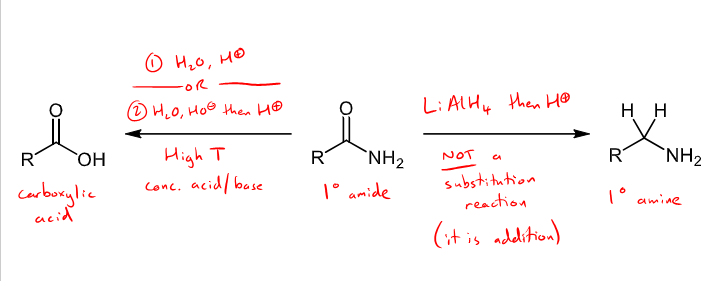
What reactions (2) with what conditions can amides undergo?
only undergo a couple of reactions as they are least reactive of the carboxylic derivatives as the carbonyl is a poor electrophile
hydrolysis - water and H+ or base hydrolysis with water, OH- and then H+, under a high temp with a concentrated acid/base to form a carboxylic acid
Reducing agent addition reaction - LiAlH4 then H+ added to form a primary amine