Chem Prac H
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
State when to use initial rate and graphical method
Use initial rate method if there is a colour change or precipitate formed (something observable)
Use titration or graphical method if one of the reactants or products can be determined by titration
Describe planning for kinetics general (first appearance of colour)
General:
Use a 10 cm3 measuring cylinder to transfer 2.0 cm3 of FA 1 into a conical flask.
Use a 50 cm3 measuring cylinder to transfer 50.0 cm3 of FA 2 into the conical flask.
Use a separate 50 cm3 measuring cylinder to measure out 50.0 cm3 of FA 3.
Quickly pour FA 3 into the conical flask while simultaneously starting a stopwatch.
Swirl the flask gently for about 5 seconds to ensure thorough mixing.
Stop the stopwatch at the first appearance of the brown colouration.
Record the time taken (t) to the nearest second in the table.
Describe planning for kinetics general (cross disappearing)
Cross thing:
Stir the contents in the beaker thoroughly with a glass rod for about 10 s.
Stop stirring and view the cross from above the beaker.
Stop the stopwatch as soon as the cross disappears or is obscured.
Record the time taken.
Repeat steps 1 to 6 for the other experiments using volumes of FA 4, FA 5 and water shown in the table.
Describe planning for kinetics graphical method
Using a balance, weigh accurately 5.0 g of ammonium chloride, FA 1, into a dry weighing bottle and record the mass of the weighing bottle and FA 1.
Place a dry polystyrene cup in a 250 cm3 beaker.
Use a 100 cm3 measuring cylinder to add 50 cm3 of deionised water into the polystyrene cup.
Stir the water in the polystyrene cup with the thermometer and measure its temperature; this is the temperature at t = 0 min
Leave the thermometer in the cup
Start your stopwatch and take a temperature reading every 1 minute for 2 minutes.
Record your temperature readings in the space below.
At exactly 3 minutes, add FA 1 quickly from the weighing bottle into the polystyrene cup.
Stir the mixture thoroughly. Do not read the temperature at 3 minutes.
Continue to stir and record the temperature every 1 minute from 4 minutes to 10 minutes in the space below.
Reweigh and record the mass of the weighing bottle after the transfer, which may contain some residual FA1
Record the mass of FA 1 used.
Plot a graph of temperature (y-axis) against time (x-axis)
Draw a straight line of best-fit for the points before t = 3 min.
Draw a second straight line of best-fit for the points after t = 3 min. Extrapolate both lines to t = 3 min.
Read off from your graph the minimum temperature and the maximum temperature at t = 3 min
Record these values in the spaces provided below the graph and determine an accurate value for the temperature change at t = 3 min
Why must weighing bottle be dry
To prevent contamination of the ammonium chloride. Some of the FA 1 will dissolve in the water before the experiment starts -> calculated enthalpy change will be inaccurate
Why is the polystyrene cup placed in a beaker
To prevent the polystyrene cup from toppling over when the solution is stirred. The air that is trapped between the beaker and the polystyrene cup serves as an insulation to reduce heat transfer.
Draw kinetics graph
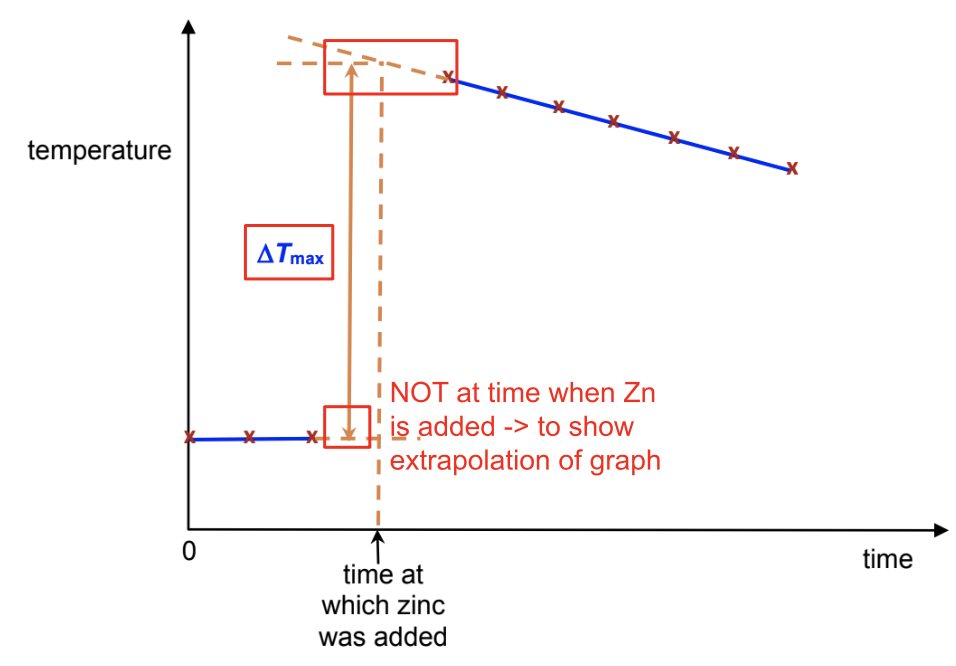
Why is graphical kinetics method more accurate
The graphical method is more accurate as it takes into consideration the heat
gained from the surroundings
Describe enthalpy change of combustion of fuel experiment
Use a measuring cylinder to introduce 100 cm3 into the copper calorimeter.
Place the thermometer in the water and record its initial temperature.
Place some XXX into the oil lamp (also known as spirit lamp)
Put a wick into it and weigh the spirit lamp and its contents. Record the mass, m1
Place the oil lamp under the calorimeter and light up the spirit lamp
Stir the water gently with the thermometer. (Do not let the thermometer touch the base of the copper calorimeter)
When the temperature of the water has increased by about 5 oC, extinguish the spirit lamp.
Weigh the oil lamp and its contents immediately. Record the mass, m2
Record the highest temperature of the water and calculate the ∆T
Calculate the mass of XXX used (i.e. m1 – m2)
Repeat the experiment to get reliable results