hematology exam 3 review disorders of iron and heme metabolism + IDA, SA, and ACI
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
Iron is an essential element for all living organisms Because it:
Cellular growth, oxygen transport, and proliferation of
RBCs
When oxygen tensions drops,
Receptors in the kidney can sense the change in oxygen tension/concentration- making more EPO to release into the bone marrow
When EPO is released into the bloodstream, the bone marrow responds to this by
allowing for the early release of reticulocytes, increases the number of mature erythrocytes, and increases the rate of maturation of erythroid precursors - so there’s accelerated release effect into peripheral blood.
Reticulocyte Production Index, a calculation which indicates
whether or not bone marrow is responding adequately to anemia.
Normal bone marrow response can increase erythropoiesis by? And for how long would it take for compete response to occur?
6-8 fold; a full week
disorders that present with an increase in circulating RBC (increased Hct)
Erythrocytosis and Polycythemias
disorders that present with a decrease in circulating RBCs (decreased Hct)
Anemias
Physiologic Adaptations in Anemia, how does it affect the Oxy. dissociation curve?
Hypoxia occurs due to decrease in O2-carrying capacity.
Diffusion causes ↑ O2 release by Hgb- shifts the oxy dissociation curve to the right. (decreased O2 affinity of Hgb)
The body increases 2,3-DPG production to further help with off-loading oxygen.
Via selective vasoconstriction, RBCs are rerouted to areas where there is highest oxygen demand, and this requires increased cardiac output. In severe anemia, this can even cause tachycardia. (inc heart rate)
With anemia, what is its relationship with vasoconstriction?
RBCs are rerouted to areas where there is highest oxygen demand, and this requires increased cardiac output. In severe anemia, this can even cause tachycardia. (inc heart rate)
Absolute anemia definition and examples
true decrease in red cell mass; impaired RBC production, blood loss or accelerated RBC destruction or hemolysis
Relative anemia definition and examples
apparent decrease in red cell mass not from true hematologic disorder; fluid shifts (ex in pregnancy) or diluted blood from (IV)
Retic count test: Schilling’s test
tests for B12 in urine
CBC tests
Hgb, Hct, MCV, MCH,MCHC, RDW
To test for anemia, a high reticulocyte counts indicates that there is
shortened RBC survival
A low reticulocyte count when testing for anemia indicates what is happening to RBCs
decreased RBC production
Normal hemoglobin values for adults
males: 13.5-18.0 g/dL and Females 12.0-15.0 g.dL
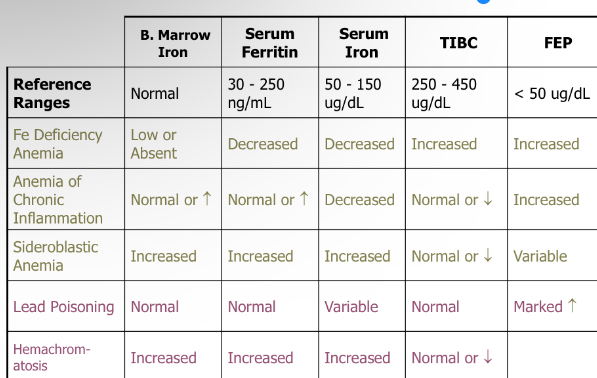
Anemia types: Acute blood loss, hemolytic anemias, early stage aplastic anemias, myelopthisic anemia, stem-cell related anemia display what RBC morphology?
normocytic, normochromic
what types of anemia conditions would normocytic and normochromic RBCs indicate?
Acute blood loss, hemolytic anemias, early-stage aplastic anemias, myelophthisic anemia, stem-cell related anemia
Macrocytic and normochromic RBCs would indicate what anemia conditions?
MCV=high, MCH=high, MCHC=normal
megaloblastic anemia, anemia of liver disease, chronic aplastic anemia, acute hemolytic anemia w/ shift retics
Anemia of chronic inflammation has what type of RBC morphology?
microcytic and normochromic; MCV=low, MCH= normal , MCHC= normal
microcytic and normochromic; MCV=low, MCH= normal , MCHC= normal
indicates what anemia condition?
Anemia of chronic inflammation
iron deficiency anemia, thalassemia, lead poisoning, porphyrias, sideroblastic anemia would show what RBC morphology
microcytic, hypochromic; MCV,MCH, MCHC= all are low
Average adult has total body iron content of
3500- 4000 mg
2/3 of iron is stored as
Hemoglobin
1/3 iron found in
Bone marrow, liver, and spleen
Stain used for iron
Prussian blue stain
Minimum daily requirement (MDR) of iron for adults is
1mg
Daily requirements of iron are affected by
Chronic blood loss
Increased need
Inadequate intake
Serum Fe levels. Exhibit both
Monthly and diurnal (daily) variation, so draw labs for Fe studies first thing in am, & fasting!
Apotrensferrin
Iron not bound to transferrin (review to check)
Iron is ingested in foods as
Fe3+ Ferric
Total Iron Binding Capacity (TIBC)
total amount of iron that can be bound by transferrin. It is effectively an indirect measurement of transferrin concentration.
Normal range for TIBC?
NR = 250- 400 ug/dL
Most common type of anemia
Iron deficiency anemia (IDA)
When does IDA occur?
whenever Fe loss or need exceeds Fe intake
conditions when IDA could develop?
States of increases physiologic demand
Inadequate intake
Chronic blood loss
Chronic blood loss could occur in the following, leading to IDA:
a. Increased menstrual flow
b. Chronic bleeding - gastric ulcers, GI carcinoma
c. Frequent blood donation
d. Chronic hemolysis - malaria, paroxysmal nocturnal hemoglobinuria
e. Hookworm infestation - Necator americanus, Ancylostoma duodenale
Common symptoms of all SEVERE anemias:
dyspnea (shortness of breath) & dizziness
Characteristic IDA symptom - pica, what is pica?
(the persistent, compulsive desire to eat a single food, or more commonly, a nonfood item
laundry starch, dirt, clay, cigarette butts, ice, etc
Characteristic IDA symptom – koilonychia, what is it?
flattened, spooned fingernails
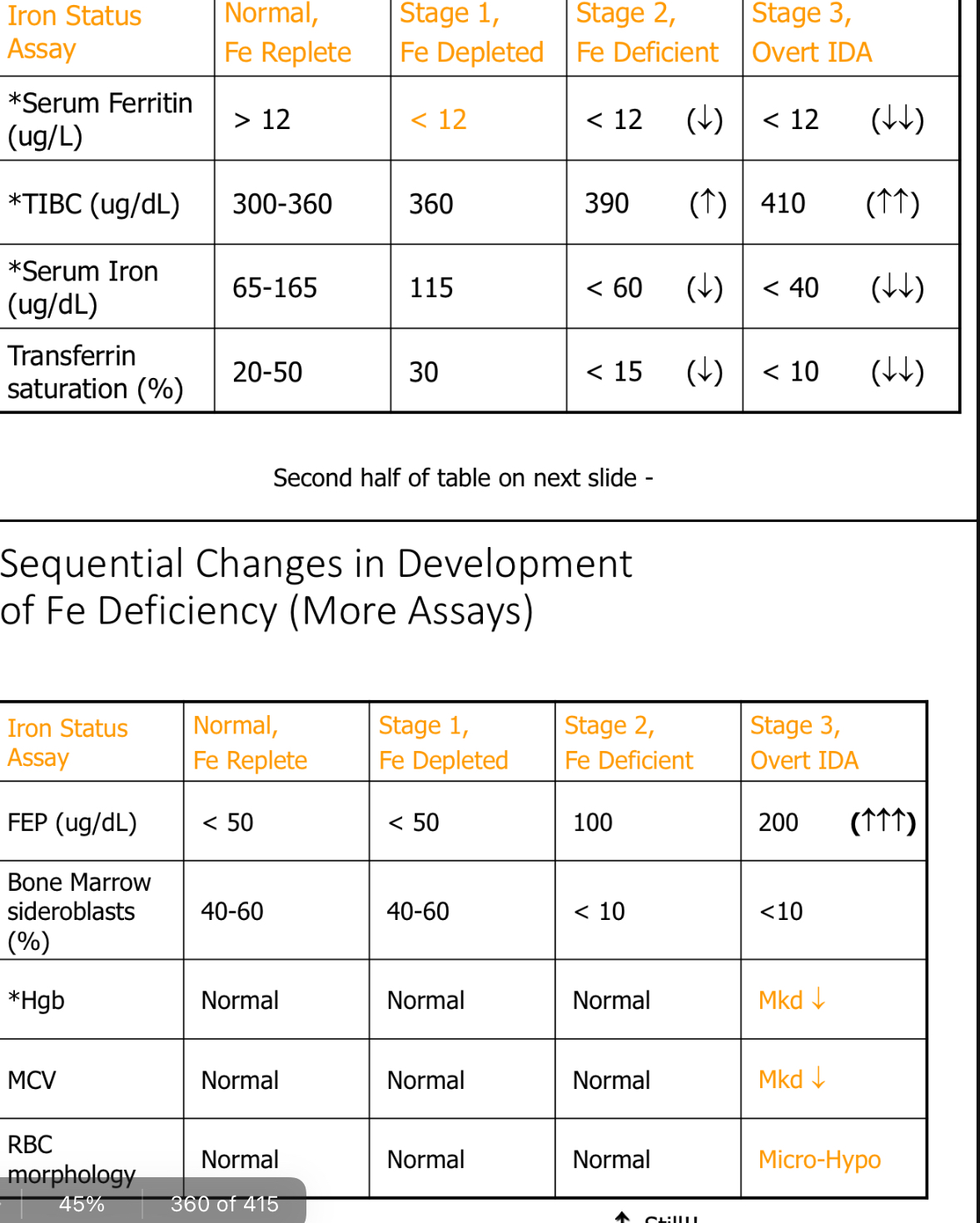
Moderate aniso with ↑ RDW; why?
Microcytes
Heme lab findings in IDA?
Microcytic Hypochromic anemia
With IDA, Blood smear shows
poikilocytosis w/ codocytes, elliptocytes or ovalocytes, and dacrocytes
Developing red blood cell
Sideroblasts
Sequential Changes in Development of Fe Deficiency (More Assays)
Paste chart
IDA treatment
First, treat underlying contributing cause
>Give oral Fe supplements & treat symptoms as needed. (Transfusions only given in life- threatening emergencies!)
>Retic count should increase within 48_h_rs_., reaching maximum in ~10 days.
>Dimorphic population of RBCs becomes evident as new “Fe normal" RBCs increase in #, while the old “Fe deficient" RBCs die off.
Dimorphic population is referred to as?
A variation in size (ie. anisocytosis)
Anemia of Chronic Inflammation
The 2nd most common anemia - associated with chronic infections
Central feature of anemia of chronic inflammation is
sideropenia (iron deficiency) with abundant iron stores
Anemia from chronic inflammation is NOT due to
bleeding, hemolysis, or bone marrow replacement by cancer or a leukemia
What does cause anemia of chronic inflammation?
Appears to be due to the body withholding Fe from inflamed cells, pathogenic microbes, & tumor cells, in an attempt to starve them to death!
Hepcidin function
Decreasing iron secretion
Main hormone responsible for anemia of chronic inflammation
Hepcidin
What produces Hepcidin?
hepatocytes, macrophages, intestinal enterocytes
Hepcidin is also an acute-phase reactant, so during inflammation (unrelated to iron levels), there is
a decrease in iron absorption and macrophages retain iron.
Most important acute phase reactant in ACI
Hepcidin
Lactoferrin (acute-phase reactant) is a
iron-binding protein in neutrophilic granules (importance in phagocytosis). During inflammation, lactoferrin is released into plasma and scavenges available iron. RBCs do not have lactoferrin receptors
Ferritin (acute-phase reactant) also binds
iron in plasma. RBCs lack ferritin receptors.
Treatment of ACI
First, treat the underlying condition
Can give EPO, but must also supply iron supplements
concurrently
Taking iron supplements (without EPO) could be harmful, even fatal
With anemia of chronic infection, what is the main concern regarding iron?
Iron availability / release
Clinical Symptoms of ACI
Decreased serum iron and % Tf saturation
Normal to increased ferritin levels
TIBC decreased
ZPP increased
TfR (transferrin receptors) are normal (increased in IDA)
Also includes symptoms of the primary disorder.
Lab Findings in ACI
Mild to mod. normo-, normo- anemia (but can sometimes look micro-, hypo-) (depending upon on how blocked Fe release is.)
No reticulocytosis (body somehow knows it isn’t really Fe deficient)
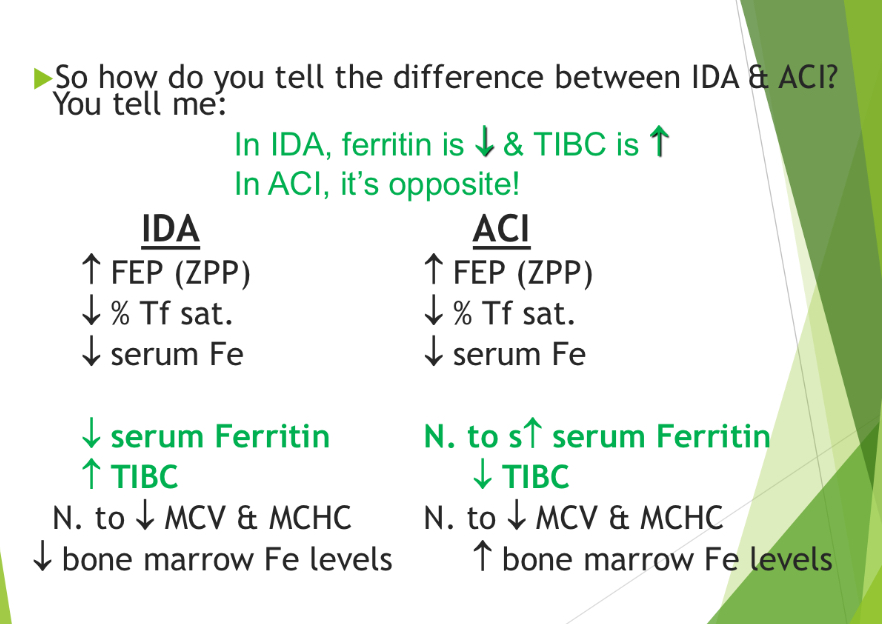
how do you tell the difference between IDA & ACI?
IDA- ferritin dec and TIBC inc (opposite for ACI)
ACI- inc bone marrow Fe lvls
IDA dec bone marrow Fe lvls
What type of patient population would
be most likely at risk for ACI
Individuals who have been hospitalized for a long time
Sideroblastic anemias - diverse group of anemias characterized by
hypochromic anemia
ineffective erythropoiesis
an increase in serum and tissue iron
the presence of ringed sideroblasts in the bone marrow.
Characteristic ringed sideroblast In SA
sideroblasts in which iron is accumulated in the mitochondria that surround the nucleus)
-Visible w/ Prussian blue stain
abnormalities of the enzymes regulating heme synthesis In SA
Identified deficiencies of δ-ALA synthetase and uroporphyrinogen decarboxylase
*Delta ALA synthetase requires pyridoxine/pyridoxal (Vitamin B6) as a coenzyme.
Sideroblastic anemia (SA) is caused by
inability to make protoporphyrin IX, the body has adequate iron which enters normoblasts, but is unable to incorporate it into hemoglobin
When patient has lots of iron but their body doesn’t have the enzymatic capacity to use it, what anemia is this
Sideroblastic anemia
Sideroblastic Anemias (SA) etiology
Etiology – typically follows exposure to drugs or toxins (i.e. antitubercular therapy, ethanol [most common culprit*], chloramphenicol, alcohol, lead, and chemotherapeutic agents
How to identify Sideroblast on bone marrow smear
Use the Prussian blue stain
Siderocyte
mature RBC in peripheral blood containing free Fe granules (aka. siderotic granules or Pappenheimer bodies)
Hereditary SA involves a defect of
δ-ALA synthetase
Idiopathic definition
Unknown cause
80% of iron carried by transferrin is used for ?
synthesis of heme
how many atoms of ferric iron can bind to one transferrin molecule?
2
relationship between iron and the stomach?
The low pH of the stomach reduces ferric iron (Fe3+) to ferrous iron (Fe2)
Gut mucosal cells work to absorb iron when it leaves the stomach by
oxidizing ferrous iron (Fe2+) BACK to ferric form (Fe3+), where it enters the bloodstream then is transported by transferrin for distribution to body tissues or stored in the spleen/liver/bone marrow
Why is hemosiderin not as used compared to ferritin for iron storage?
Hemosiderin is not water soluble and less readily available in the body
Fe without ferritin is what?
Apoferritin
When Transferrin concentration increased, what happens to iron? and why?
Iron decreases so that the body can scavenge up any available Fe atoms
Because of the inverse relationship between Transferrin and Fe, what happens to TIBC if a patient has Iron deficiency anemia (IDA)?
TIBC increases
Porphyrias are a
group of disorders due to an impaired production of heme; Results in ineffective hematopoiesis and sideroblastic anemia
Porphyrias: When an enzyme is missing, the products from earlier stages in the pathway
accumulate in the blood and may be excreted in urine or feces
Acute Intermittent Porphyria: most common porphyria
Missing enzyme is porphobilinogen deaminase
Massive build up of porphobilinogen and ALA in urine
What are Pappenheimer bodies?
Iron
Lead (Pb) Intoxication leading to SA
Acquired condition,
May be chronic or acute
Occurs in both adults (occupational exposure) & children (excessive exposure to pre-1970 Pb- containing paint, which tastes? Sweet
Improperly glazed cooking pottery & vehicle emissions (even in soil near highways) can affect all age groups.
Lead (Pb) affects three major tissues
renal, hematopoietic, & central nervous system.
Pb inhibits activity of 3 enzymes in heme synthesis pathway, which are
PBG synthase
Coproporphyrinogen oxidase
Heme synthase/synthetase (aka. Ferrochelatase.) Last enzyme in pathway!
Lead Intoxication Lab Findings
Mild to moderate micro-, hypo- anemia in chronic exposure (but can be normo- normo- otherwise).
Normal serum Fe, ferritin, & TIBC; but increased FEP! (Since inability to link heme & Fe together at the last step in the pathway causes an over- accumulation of protoporphyrin IX.)
Hallmark is basophilic stippling (but not always!)
Lead intoxication interferes with
Incorporation of iron in heme
Hemochromatosis
An inappropriate (or abnormal) accumulation of excess iron, resulting in tissue damage
Excess iron is stored in the liver, heart, and pancreas – leading to damage of these organs
This can cause bronze skin pigmentation
Hereditary Hemochromatosis (HH)
One of the most frequent genetic diseases in
Northern European Caucasians (1:300)
Tf Receptors appear to be permanently “turned on”. (It may also be due to mutant hepcidin – the patient cannot become hypoferremic when needed.)
Patients absorb normal amount of iron, but they transport more of it into the plasma
Acquired Hemochromatosis
occur secondary to other inherited hemolytic anemias
Common characteristics are anemia, ineffective erythropoiesis, and iron overload
Usually have multiple transfusions...leads to increased iron storage due to no mechanism for iron excretion
Hemochromatosis: When excess iron is present:
Increased ferritin
Increased hemosiderin
Free iron increases (ferrous form)
Ferrous iron + oxygen = superoxide & free radicals....leading to cell death
desferoxamine
Treatment option for hemochromatosis that is chelating agent used to reduce iron stores).; not as effective as simple bloodletting and is used when hemoglobin is less than 10 g/dL
Differentiation of Fe Metabolism Disorders