AP Chem Unit 5
5.0(2)
Card Sorting
1/21
Last updated 3:02 PM on 11/16/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
1
New cards
k unit for zero order
M/s
2
New cards
k unit for first order
s-1
3
New cards
k unit for second order
M-1 s-1
4
New cards
k unit for third order
M-2 s-1
5
New cards
increasing temperatures ________ collision rate
increases
bc it allows for more particles to reach the activation energy threshold
bc it allows for more particles to reach the activation energy threshold
6
New cards
breaking particles into smaller pieces ______ collision rate
increases
bc it increases surface area
bc it increases surface area
7
New cards
what do catalysts do?
provide a different reaction path that lowers the required activation energy
8
New cards
increasing molecularity _______ collision rates
decreases
bc it makes it harder to get the perfect collision
bc it makes it harder to get the perfect collision
9
New cards
endothermic
products are at higher energy level than reactants
uphill is endo
uphill is endo
10
New cards
exothermic
products are at lower energy level than reactants
downhill is exo
downhill is exo
11
New cards
which step in the mechanism has the highest activation energy?
slow step
12
New cards
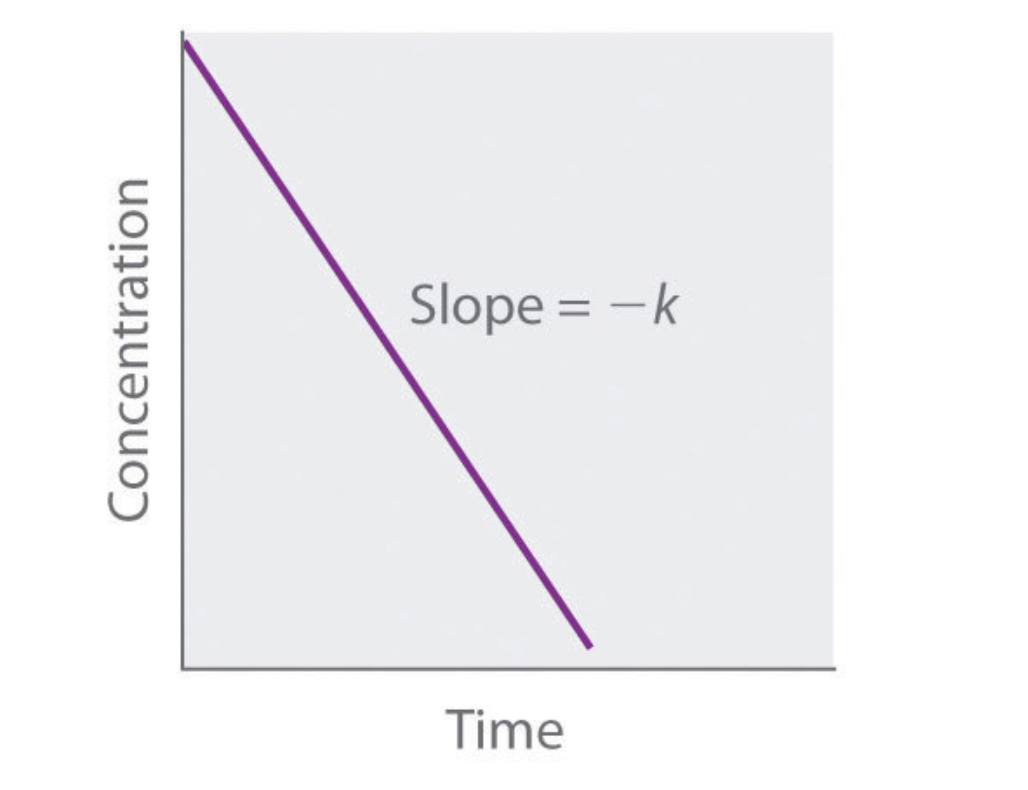
what order is this graph?
zero order
13
New cards
half life formula
initial concentration/2^x = leftover concentration
14
New cards
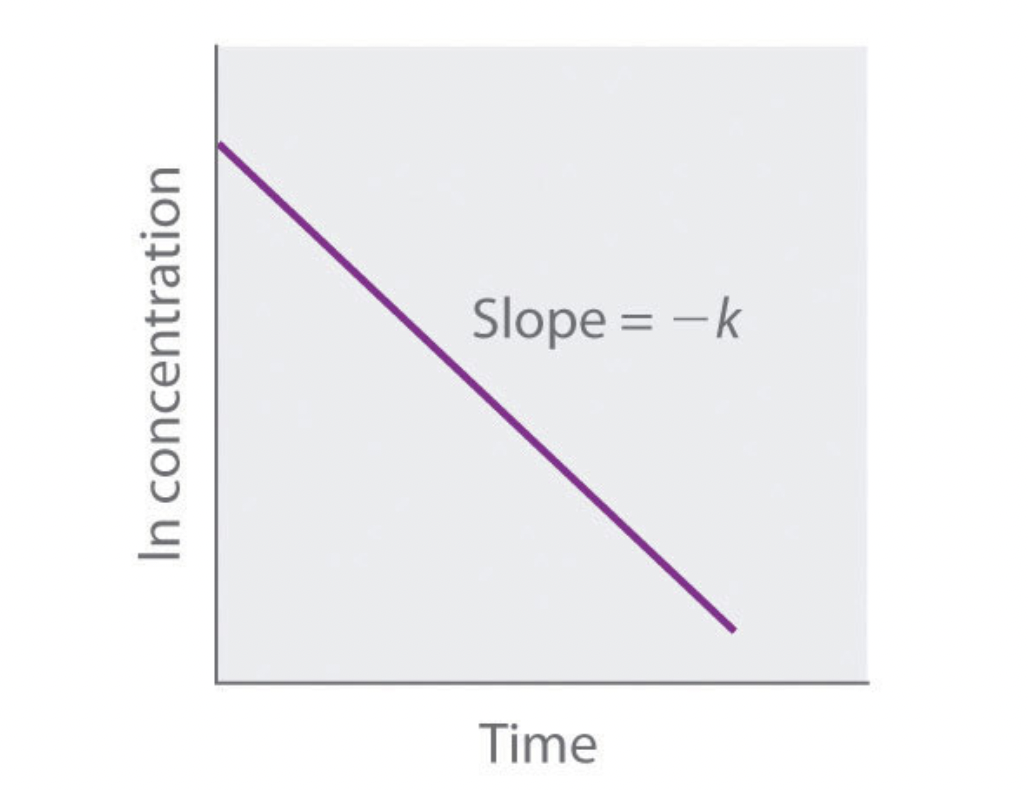
what order is this graph?
first order
15
New cards
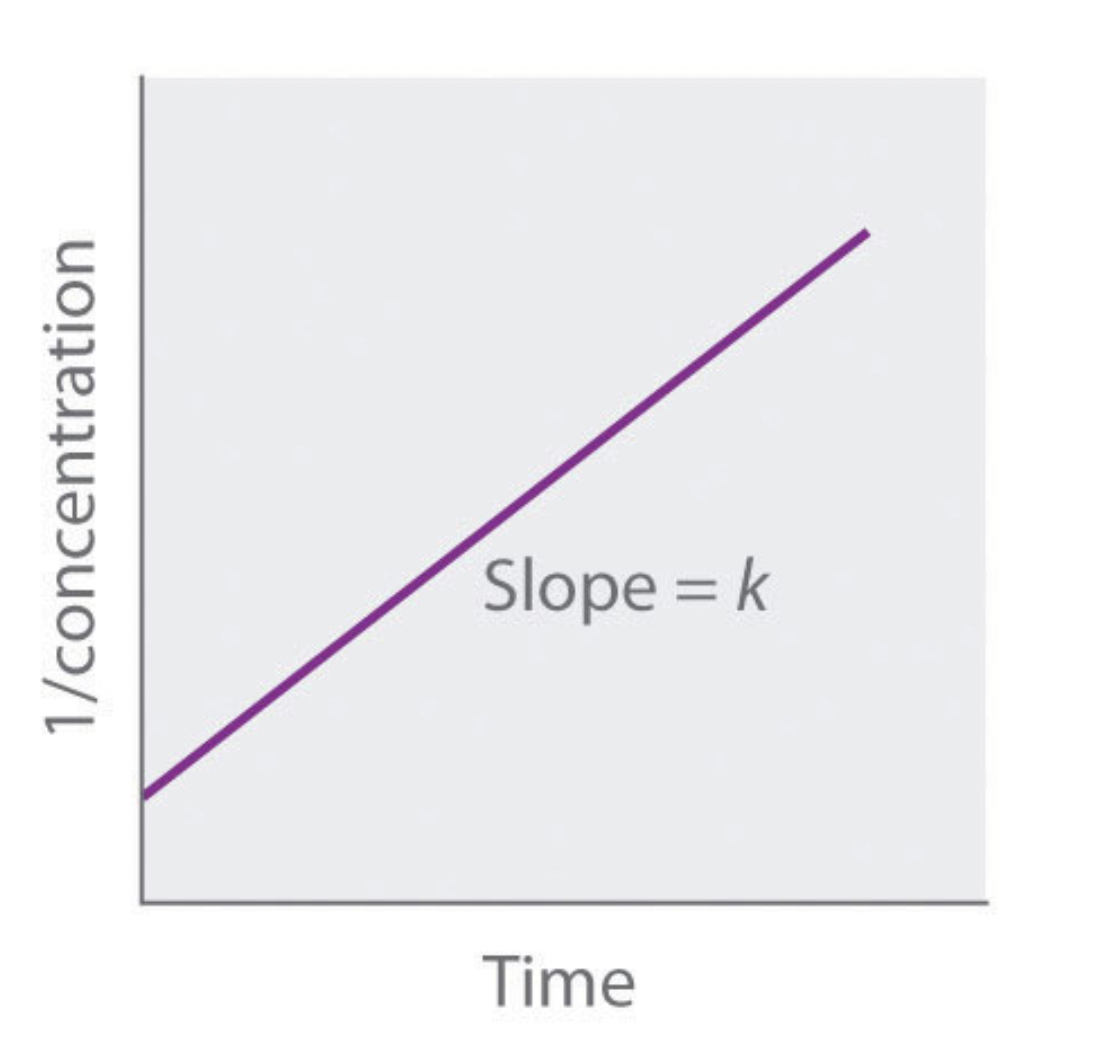
what order is this graph?
second order
16
New cards
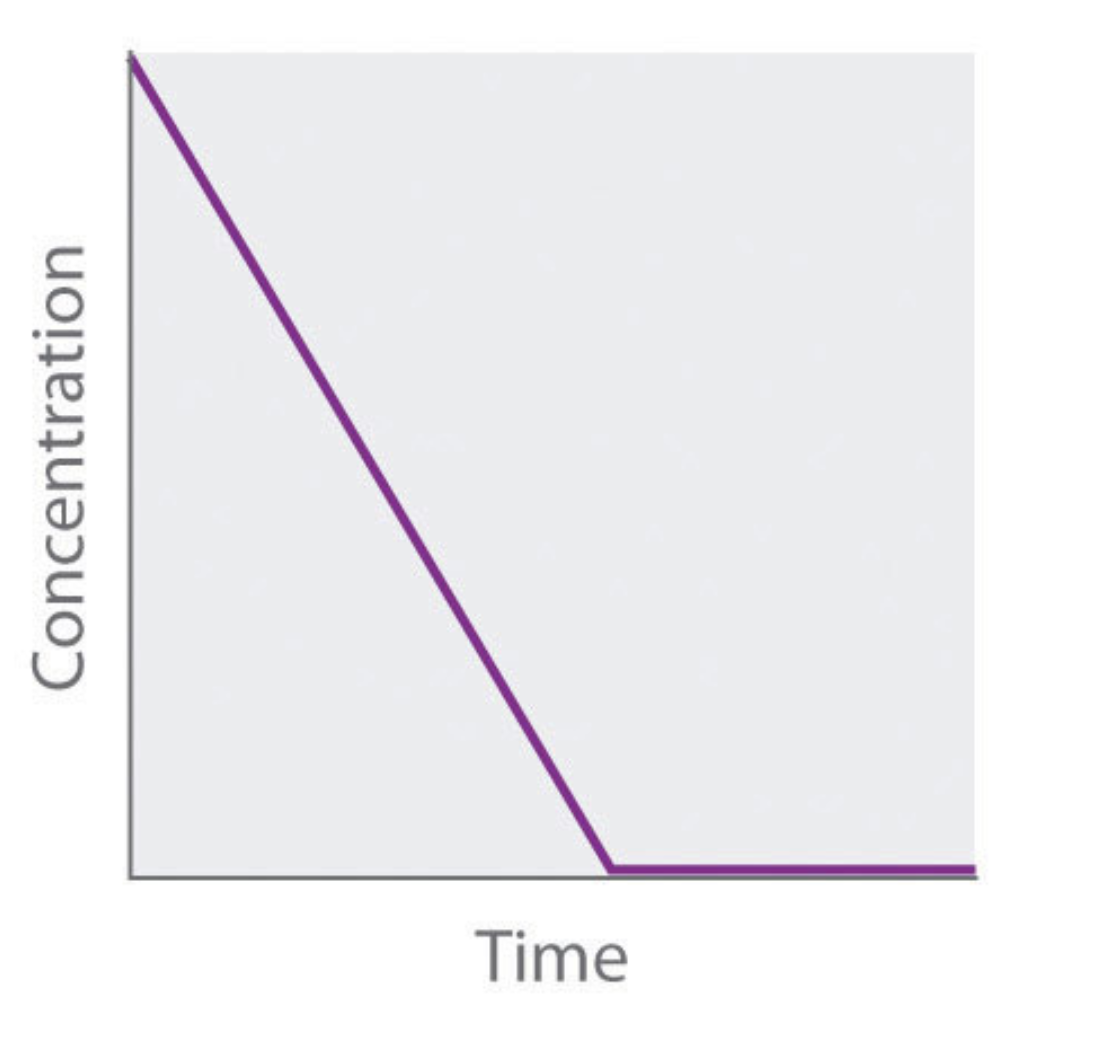
what order is this graph?
zero order
17
New cards
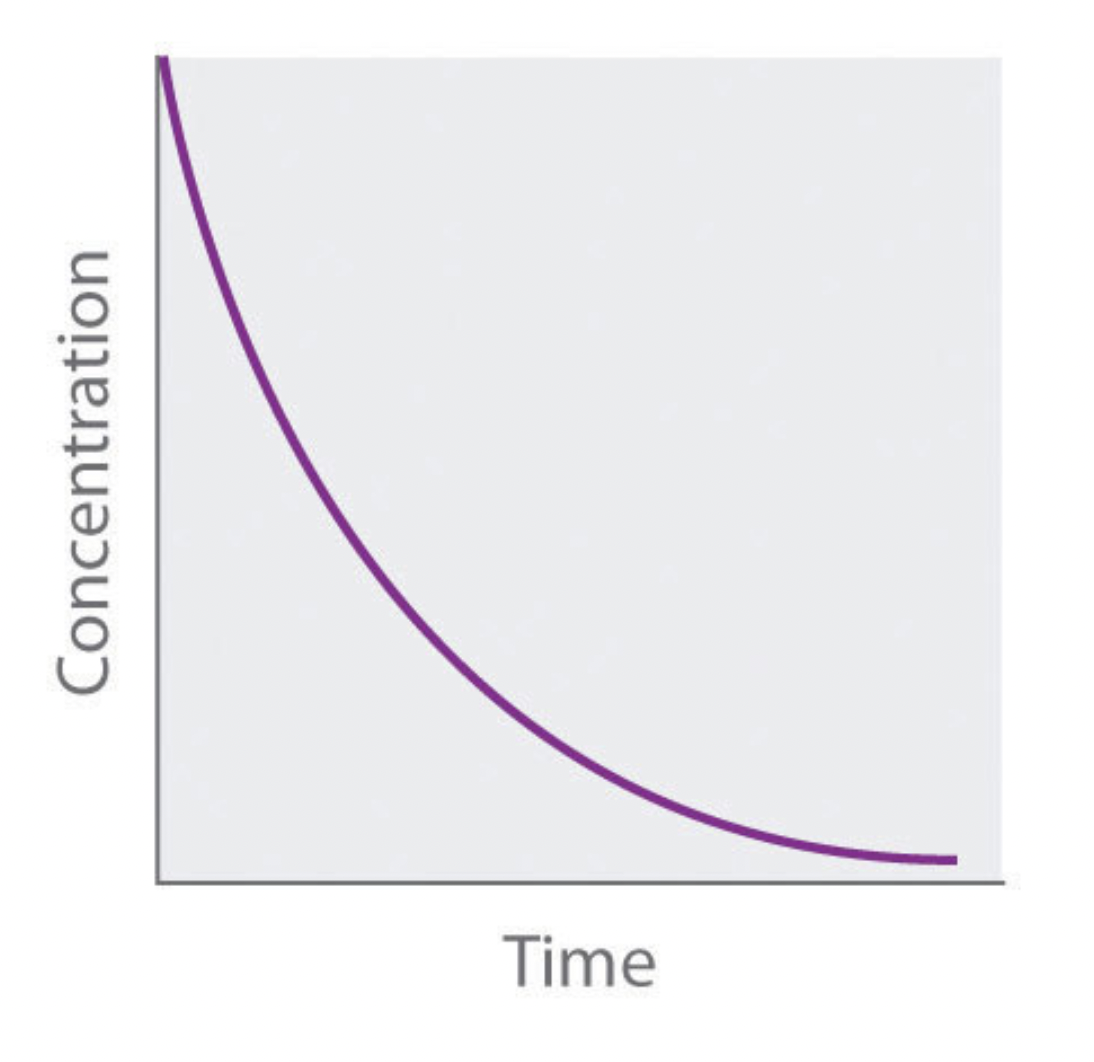
what order is this graph?
first order
18
New cards
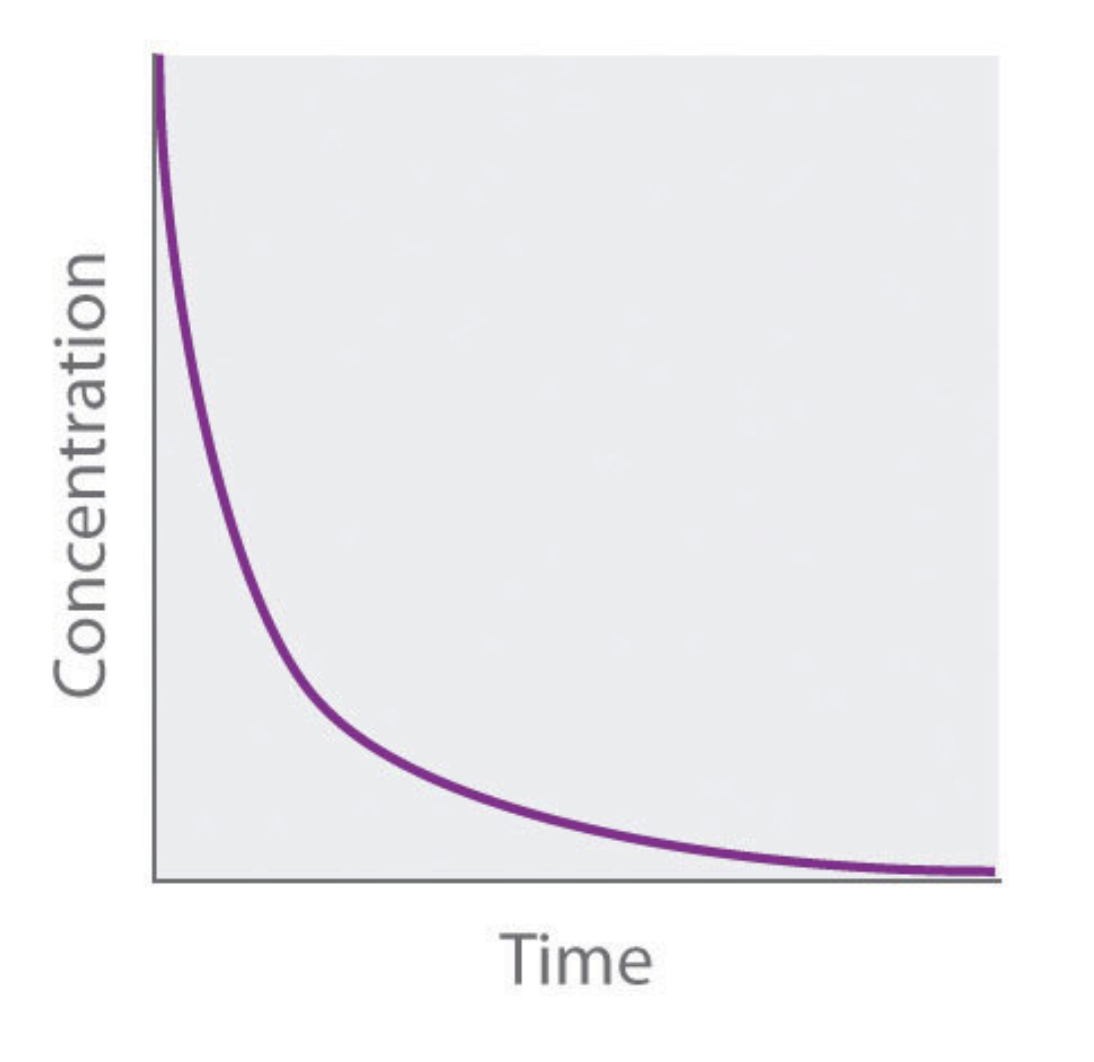
what order is this graph?
second order
19
New cards
what does the Arrhenius equation reveal?
K increases with increasing temperature
K decreases with increasing activation energy
K decreases with increasing activation energy
20
New cards
zero order equation
[A]t – [A]0 = -kt
21
New cards
first order equation
ln[A]t – ln[A]0 = -kt
22
New cards
second order equation
1/[A]t – 1/[A]0 = kt