Chem Polarity Vsepr and IMFs
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Nonpolar electron negativity difference
0-0.5
Polar electron negativity difference
0.5-2
Most times a linear lewis structure means
nonpolar
most times a bent lewis structure means
polar
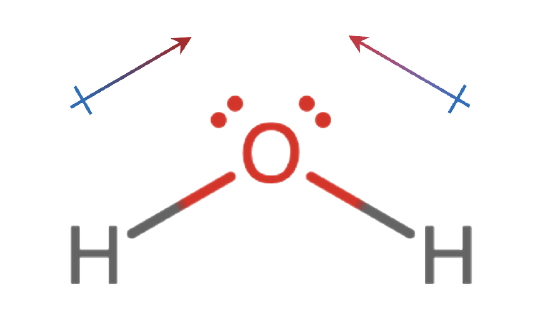
Where does the pole start
Partial Positive end
Intermolecular forces
Forces that bind atoms together to form compounds
Covelent bonds
chemical bonds between atoms where electrons are shared
Imf Influences
phase changes and surface tension
Phase changes
Melting point, evaporation, and freezing point
Surface tension
The property of a surface of a liquid that allows it to resist an external force
Ion-Ion
Forces between ionic compounds (opposites attract)
Ion-Dipole
The dissolving of compounds
Dipole-Dipole
An attraction between oppositely charges regions of a polar molecule
Hydrogen Bond
The dipole–dipole interactions experienced when H is bonded to N, O, or F are unusually strong.
London Dispersion Force
are attractions between an instantaneous dipole and an induced dipole.
The Larger Molecule the BLANK LDF’s
stronger
In dipole-dipole interactions, the more polar the molecule
the higher the boiling point
If two molecules are of comparable size
and shape, BLANK interactions
will likely be the dominating force.
dipole–dipole
If one molecule is much larger than
another, BLANK will likely
determine its physical properties.
dispersion forces
In the AXE formula X represents
number of single line pair
In the AXE formula E represnt
number of lone pairs
The atom with the higher electronegativity has a
partial negative charge
The atom with lower electronegativity has a
partial positive charge